Translate this page into:
Pulmonary Renal Syndrome due to Atypical HUS in a Young Gentleman
Address for correspondence: Dr. Jasmine Sethi, Department of Nephrology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. E-mail: jasmine227021@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
A 20-year-old gentleman with no previous comorbidities presented with decreased urine output, anasarca, and shortness of breath of 1 week duration. There was no history of fever, hematuria, frothuria, hemoptysis, skin rash, joint pains, or drug abuse. The patient had undergone open reduction and internal fixation for right tibial fracture 2 days before symptomatology. The blood pressure recorded before surgery was 130/80 mmHg, and his serum urea was 34 mg/dl and serum creatinine was 0.8 mg/dl. At admission, examination revealed blood pressure of 160/100 mmHg, severe pallor, tachycardia, tachypnea requiring oxygen supplementation at 15L/min, and bilateral crepitations in the infra-scapular and infra-axillary areas. Lab evaluation revealed anemia with a hemoglobin of 8.5 g/dl, leukocytosis (23 × 109/L), platelet count of 540 × 109/L, serum creatinine of 8 mg/dl, serum albumin of 3.4 g/dl, and urine protein excretion of 1.6 g/day with microscopic hematuria. Hemoglobin showed a progressive decline to a nadir of 4.7 g/dl on day 4 of admission with no evidence of hemolysis. He was initiated on hemodialysis and was transfused three units of packed red cells. Ultrasonography showed normal size kidneys, and chest imaging revealed bilateral dense airspace opacities throughout both lungs with peripheral sparing suggestive of diffuse alveolar haemorrhage (DAH) [Figure 1a]. Serologies including antinuclear, anti-glomerular basement membrane antibody, antinuclear cytoplasmic antibody, viral serologies, and serum cryoglobulins were negative. Evaluation of the complement system revealed low C3 of 72 mg/dl (reference range 90–180) with normal C4. The patient underwent renal biopsy that showed features of acute and subacute glomerular and vascular thrombotic microangiopathy (TMA) on light microscopy [Figure 1b]. Direct immunofluorescence was negative for immunoglobulins and complements. Given the clinical picture of pulmonary renal syndrome, the patient was empirically initiated on pulse methylprednisolone and plasmapheresis. A possibility of fat embolism syndrome (FES) was also considered; however, the normal neurological status, absence of skin rash, and kidney biopsy findings excluded FES. Post five sessions of plasmapheresis with fresh frozen plasma as replacement and steroids, the patient improved with a complete renal recovery and resolution of DAH. Genetic testing by multiplex ligation-dependent probe amplification (MLPA) assay revealed CFH/CFHR1/CFHR3 gene duplication. Whole-exome sequencing was planned but could not be done due to financial constraints. Steroids were tapered and stopped with a serum creatinine of 0.7 mg/dl at 3 months. The patient was not given maintenance immunosuppression or eculizumab. A diagnosis of atypical hemolytic uremic syndrome (aHUS) presenting with DAH was made.
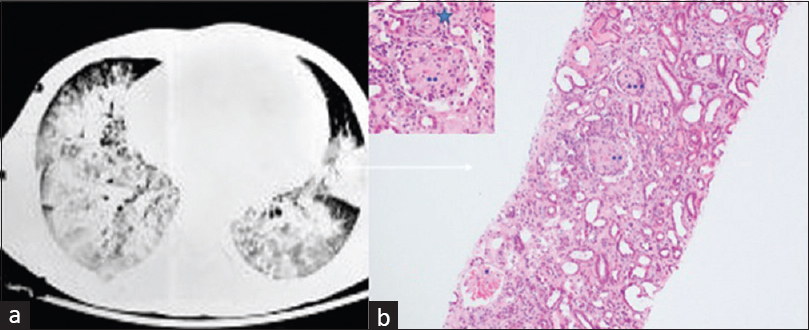
- (a) Computed tomography scan of chest showing bilateral diffuse air space opacities with peripheral sparing suggestive of diffuse alveolar hemorrhage. (b) Photomicrograph shows different stages of glomerular thrombotic microangiopathy – acute stage (*mesangiolyses), subacute (**fibrillated mesangium), and chronic (***collapse) along with vascular subacute TMA (star, inset) (hematoxylin and eosin with 20× magnification, inset 40×
aHUS is a syndrome characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute renal injury. aHUS presenting as DAH is exceedingly rare. Here, we present a young boy who presented with DAH, thrombocytopenia, and dialysis-dependent renal dysfunction with biopsy-proven glomerular and vascular thrombotic microangiopathy. The patient was managed with dialysis, steroids, and plasmapheresis. Genetic study (MLPA) showed duplication of CFH, CFHR3, and CFHR1 exons. Hence, aHUS should be considered in the differential of patients presenting with DAH.
DAH usually presents with hemoptysis, anemia, hypoxia, and diffuse alveolar infiltrates. Absence of hemoptysis should not exclude this diagnosis, as it has been reported to be initially absent in up to 33% of cases.[1] The commonest causes of pulmonary renal syndrome include ANCA-associated vasculitis and anti-glomerular basement membrane disease. However, aHUS manifesting as DAH is exceedingly rare. The cause of DAH in aHUS is unclear, and it is postulated that thrombocytopenia and thrombi formation in the pulmonary vasculature could be the contributing factors.
Hemolytic uremic syndrome is a thrombotic microangiopathy that usually presents with hemolytic anemia, thrombocytopenia, and tissue dysfunction. However, Serres et al.[2] reported normal platelet count in 44% of patients with histologically identified TMA, though only few cases have been reported without evidence of hemolysis. Sometimes, the diagnosis of TMA is made only after kidney biopsy if it is obtained for renal failure of unknown etiology. aHUS is characterized by complement overactivation, and a trigger is required to unmask the underlying complement regulatory deficiency. Our patient has antecedent surgery as a precipitating event. Other triggers identified are infections, drugs, autoimmune conditions, transplants, and pregnancy.
Here, we report the onset of aHUS in association with homozygous duplication of all CFH, CFHR3, and CFHR1 exons, which has not been previously reported with aHUS. This type of duplication leading to novel CFHR1/CFH hybrid gene encoding a fusion protein that antagonizes factor H–dependent complement regulation has been reported by Veloti et al.[3] In conclusion, aHUS presenting as DAH is rare and life-threatening. Our case highlights the importance of including aHUS in the differential diagnosis of pulmonary renal syndrome, which allows for early recognition and intervention. The investigations of patients with aHUS have become increasingly complex, and our understanding of the disease remains incomplete with new susceptibility alleles emerging worldwide.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Immune diffuse alveolar hemorrhage:Clinical presentation and outcome. Respir Med. 2017;129:59-62.
- [Google Scholar]
- Athrombocytopenic thrombotic microangiopathy, a condition that could be overlooked based on current diagnostic criteria. Nephrol Dial Transplant. 2009;24:1048-50.
- [Google Scholar]
- A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J Am Soc Nephrol. 2015;26:209-19.
- [Google Scholar]






