Translate this page into:
Spontaneous nontraumatic subarachnoid hemorrhage without cerebrovascular malformations in a maintenance hemodialysis patient
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nontraumatic subarachnoid hemorrhage (SAH) in a dialysis patient is an uncommon occurrence and is often associated with high mortality. We report for the first time in India, a case of spontaneous nontraumatic, nonaneurysmal SAH without any cerebrovascular malformation in a maintenance hemodialysis patient, following a session of hemodialysis. The dialysis prescription needs to be modified in these patients, in order to prevent worsening of cerebral edema and progression of hemorrhage. Where available, continuous forms of renal replacement therapies, with regional anticoagulation seem to be the best option for such patients, till neurologic stabilization is achieved.
Keywords
Anticoagulation
end-stage renal disease
maintenance hemodialysis
nontraumatic subarachnoid hemorrhage
Introduction
Cerebrovascular accident (CVA) is the third most common cause of death in patients on dialysis.[1] Alterations in cerebral blood flow during dialysis as well as use of anticoagulation during dialysis have been speculated to contribute to hemorrhagic stroke, but clinical evidence supporting the same is conflicting. Overall incidence of CVA in dialysis population is variously reported to be 17.2–33/1000 patient/years, with ischemic stroke being the most common type.[23] Nontraumatic subarachnoid hemorrhage (SAH) in the absence of cerebral aneurysms is rarely encountered in dialysis population and management of SAH in dialysis patients is not standardized. Nontraumatic, nonaneurysmal SAH in hemodialysis patients seems to be under-reported; we could not find any previous case reports. SAH is associated with poor outcome in patients on dialysis, and it is important to modify the dialysis prescription in such patients. We report for the first time in India, a case of spontaneous, nontraumatic, nonaneurysmal SAH in a patient on maintenance hemodialysis.
Case Report
A 45-year-old lady, diabetic for 25 years, developed end-stage renal disease (ESRD) 3 years back and was on regular thrice a week hemodialysis. For past 6 months, she was undergoing dialysis only twice a week, due to excessive fatigue following hemodialysis sessions. She had worsening hypertension and was prescribed antihypertensives, which she was not regularly taking due to significant fluctuations in blood pressure (BP) readings.
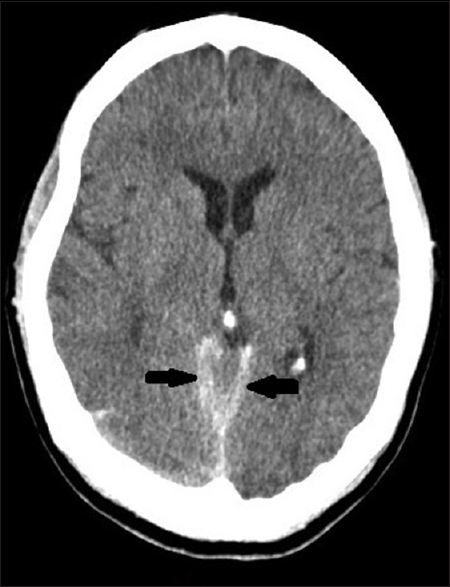
She underwent hemodialysis on 17/11/2014 from 10:00 am to 02:00 pm at her usual dialysis center. Her predialysis weight was 58 kg and postdialysis weight was 55.5 kg. She was administered 3000 units of unfractionated heparin as bolus at dialysis initiation, followed by 1000 units/h infusion during the dialysis session. Her BP at initiation was 140/80 mmHg; and at termination of the session, it was 130/80 mmHg. The dialysis session was uneventful, and she returned home. She presented with complaints of sudden onset of severe headache, which started about 4 h after the dialysis session. Headache was excruciating, with associated neck pain and multiple episodes of vomiting. At presentation to our emergency department, her Glasgow coma scale was E3V4M4, BP was 250/140 mmHg. Noncontrast computerized tomography (NCCT) brain showed SAH [Figure 1]. There was no history of fall or trauma to the head, there was no history of bleeding diathesis or intracerebral bleed in the past. She was started on labetalol infusion and BP was controlled. She was also started on nimodipine after neurosurgery consultation. Mannitol, Dexamethasone and other “anti-edema” measures were not prescribed. Within 6 h of admission, her sensorium improved, she didn’t have any focal neurological deficits. The computed tomography (CT) angiogram did not show aneurysms or arteriovenous malformations (AVM). Repeat neuroimaging was done on 4th day [Figure 2], which showed mild hydrocephalus, but no evidence of cerebral edema or progression of hemorrhage. Neurosurgeon advised conservative management.

- Noncontrast computerized tomography brain showing subarachnoid hemorrhage (pointers)
- Repeat noncontrast computerized tomography brain after 4 days, showing significant resolution of subarachnoid hemorrhage
Heparin free hemodialysis was performed after 48 h of hospitalization. She complained of headache and was having high BPs during and immediately following the initial few dialysis sessions. Her antihypertensives were adjusted during hospital stay and at the time of discharge, her BP was well controlled.
She was discharged after 2 weeks of hospitalization, with the advice to continue thrice weekly heparin-free dialysis for another 4 weeks and good BP control. Repeat neuroimaging was planned after 1-month.
Discussion
The hospitalization rate from stroke is increased 5–10 fold in dialysis population compared to general population.[4] Vast majority are ischemic in nature, with hemorrhagic stroke accounting for only about 15%; even though hypertension, bleeding diathesis associated with renal failure and administration of heparin may all increase the risk of hemorrhagic stroke. A hemorrhagic complication like SAH, with etio-pathogenesis distinct from intra-cerebral hemorrhage (ICH), seems to be distinctly unusual in dialysis patients; we could not find any previous reports of nontraumatic, nonaneurysmal SAH in hemodialysis patients from India.
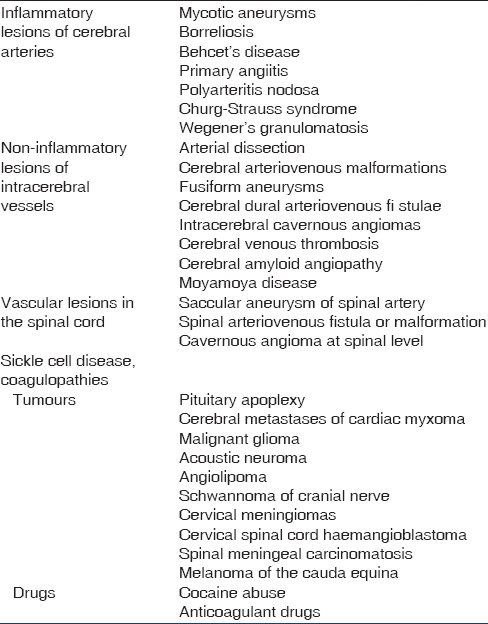
Subarachnoid hemorrhage accounts for about 5% of all causes of stroke in general population. In most populations, incidence of SAH is 6–7/1,00,000 person-years. Around 85% of all causes of SAH are due to rupture of saccular aneurysms at the base of the brain. Nonaneurysmal perimesencephalic hemorrhage account for 10% of SAH and other rare causes account for the rest 5% [Table 1]. Risk factors for SAH include genetic factors and modifiable risk factors like smoking, hypertension and excess alcohol intake. One of the most important risk factors is family history of SAH, with the first-degree relatives having 3–7 fold increased risk of SAH. Specific inherited condition like Autosomal dominant polycystic kidney disease (ADPKD) are associated with increased risk of SAH, 2% of patients with SAH have ADPKD. Associations with other inherited conditions like Ehlers–Danlos disease IV, neurofibromatosis type 1 and Marfan's syndrome are described but are rarely found in patients with SAH. Sudden increase in intramural arterial pressure may precipitate the rupture of aneurysms. Activities which can precipitate bleeding like physical exercise, sexual intercourse and straining are reported in up to 20% of cases. Nonaneurysmal perimesencephalic type of SAH is a relatively harmless type of SAH, where the extravasated blood is confined to the cisterns around the brain.[56] Compared to aneurysmal SAH, nonaneurysmal perimesencephalic SAH is associated with a milder presentation and better outcome.
Little is known about the pathogenesis as well as management of patients with SAH, who are on regular dialysis. We undertook a systematic literature search in PubMed using specific search terms [Table 2]. The search returned 194 reports, out of which five were found relevant to the search. An additional relevant publication was found on Google search. Of these 6 reports, only two specifically addressed nontraumatic SAH in dialysis population, and none specifically addressed the management of SAH in dialysis population. There seems to be a lack of medical literature on SAH in the dialysis population, despite the fact that the pathogenesis of SAH is distinct from that of other forms of intracranial bleeds like ICH and subdural hemorrhage.

Sakhuja et al., recently reported the incidence and outcomes of nontraumatic SAH in patients on maintenance dialysis in the United States, in a retrospective cohort study using the National Inpatient Sample database over a 6 years period.[7] The study excluded SAH from AVM but not SAH related to cerebral aneurysms. Out of 149,091 hospitalizations with nontraumatic SAH, 1631 (10.9%) were patients on maintenance dialysis. The incidence of hospitalizations due to SAH among patients on maintenance dialysis (73.5/100,000 population) was significantly higher than in general population (11.2/100,000 population). Unadjusted all-cause mortality was higher in the dialysis population compared to general population (38.4% vs. 21.9%) and maintenance dialysis was found to be an independent predictor of mortality following SAH. Sood et al.,[8] reported the 3 years incidence of major nontraumatic hemorrhage in elderly (>65 years) incident dialysis patients. The incidence of nontraumatic SAH was 0.1% (0.33/1000 person years). Murakami et al., previously reported the clinical features and management of intracerebral bleed and subarachnoid hemorrhage in Japanese dialysis patients hospitalized over a period of 4 years.[9] There were 36 patients with ICH and 5 patients with SAH (all etiology). Both groups of patients were initially managed with continuous hemofiltration after admission, except 2 patients with SAH, who were already on peritoneal dialysis. Once repeat CT scan showed no evidence of cerebral edema, patients were changed to hemofiltration thrice weekly. They were given Nafomostat Mesylate as anticoagulant. Three times per week hemodialysis was restarted once absence of neurologic deterioration was confirmed. A favorable outcome was reported only in two out of the five patients with SAH. Two patients died of severe SAH; one patient underwent aneurysm neck clipping on day 14 but died on day 15 due to vasospasm. None of the studies reported the incidence of nontraumtic, nonaneurysmal SAH separately, the incidence of which remains unknown in the dialysis population.
Anticoagulation associated with hemodialysis along with other risk factors like hypertension and bleeding diathesis from a uremic milieu are thought to contribute to high incidence of ICH in the dialysis population.[4] In addition to ICH, a high incidence of subdural hematoma (SDH) is also reported in the dialysis population. The incidence of SDH has been well-studied, and the incidence was found to be 10 times greater than general population. The probable pathogenic factors are volume overload leading to venous hypertension, along with uremic platelet dysfunction leading to expansion of small venous tears in dural bridging veins and use of anticoagulants.[10]
Our patient had clinical manifestations within 4 h of a hemodialysis session, and it seems possible that anticoagulation was primarily responsible for the development of SAH in her case, in the absence of other risk factors. Interestingly, she had well-controlled BP during and at the termination of the dialysis session and the high BP recorded at presentation to the emergency is likely the consequence of SAH and raised intracranial tension rather than a contributing factor for SAH.
The most striking clinical feature of SAH is often a history of unusually severe headache the onset of which is often described as “instantaneous.” This may be followed by a period of unresponsiveness and neurological deficits. Neck stiffness is a common clinical sign in SAH from any cause, but often develops hours later. Other clinical features may include projectile vomiting, seizures and coma. It may be prudent to suspect SAH in a dialysis patient who complains of a relatively abrupt onset and unusually severe headache with or without other neurological deficits. The extravasated blood in basal cisterns in SAH give a characteristic appearance on NCCT scan, and a CT scan is first line of investigation in a case of suspected SAH. It is necessary to investigate the cause of SAH in cases where it is not the result of trauma. CT Angiography is the imaging modality of choice to detect AVMs and saccular aneurysms causing SAH. Management of SAH in general population includes, general supportive measures including nursing care, nutrition, control of BP, management of fluid and electrolyte imbalances, pain control, prevention of delayed cerebral ischemia with calcium channel blockers and prevention of deep vein thrombosis and pulmonary embolism. This should be combined with definitive management of aneurysms with coiling or surgical clipping, when indicated.[5] Average case fatality rate in general population is 51%.[6] Long-term complications of SAH include late rebleeding, epilepsy, anosmia, cognitive and psychosocial dysfunction.[5]
Management of ESRD patients with ICH or SAH with raised intracranial tension is problematic. Administration of “anti-edema” measures like mannitol and loop diuretics is either not possible or unlikely to be useful. Intermittent hemodialysis sessions may result in wide fluctuations in osmolality and BP and may have adverse neurologic consequences. Continuous forms of renal replacement therapy have been shown to provide better cerebrovascular stability in patients with cerebral edema.[1112] Such therapies produce less fluctuation in serum osmolality and BP, thereby causing less change in intracranial pressure as well as cerebral perfusion pressure. In hemodialysis patients with hemorrhagic stroke, it may be better to do continuous hemofiltration rather than intermittent hemodialysis. Once neurologic stabilization is attained, they can be shifted to conventional hemodialysis. When intermittent hemodialysis is used, dialysis prescription needs to be altered to prevent rapid changes in serum osmolality and to prevent hypotension.[13] This can be done by decreasing blood flow and dialysate flow rates, choosing small surface area dialyzers, reducing dialysis time, increasing dialysis frequency and by setting higher dialysate sodium concentration. Systemic anticoagulation should be avoided in those with hemorrhagic stroke. Instead, heparin free dialysis or regional anticoagulation with Citrate or Nafomostat can be used, when available.
In our patient, we could not find any precipitating event for SAH, except probably anticoagulation during the hemodialysis session which immediately preceded the SAH. The most common cause of SAH is trauma followed by intracranial aneurysms. In the present case, the etiology could be venous bleed (from anticoagulation) as happens in nonaneurymal perimesencephalic SAH. In the absence of facilities for continuous renal replacement therapy and hemodiafiltration, we resorted to cautious intermittent hemodialysis done on alternate days, with minimum ultrafiltration and sodium profiling during dialysis session in an attempt to improve BP control during the sessions. The hemodialysis sessions were largely uneventful, except for high BP records despite precautions. She was asymptomatic and was discharged within 2 weeks of hospitalization. The limited nature of SAH in our patient might account for the good outcome in her.
Source of Support: Nil
Conflict of Interest: None declared.
References
- US Renal Data System: USRDS 2007 Annual Data Report. Atlas of Chronic Kidney Disease and End. Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007.
- [Google Scholar]
- Okawa Dialysis Study (OKIDS) Group. Clinical demographics and long-term prognosis after stroke in patients on chronic haemodialysis. The Okinawa Dialysis Study (OKIDS) Group. Nephrol Dial Transplant. 2000;15:1808-13.
- [Google Scholar]
- Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol. 2003;14:2623-31.
- [Google Scholar]
- Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603-9.
- [Google Scholar]
- Subarachnoid haemorrhage: Diagnosis, causes and management. Brain. 2001;124(Pt 2):249-78.
- [Google Scholar]
- Nontraumatic subarachnoid hemorrhage in maintenance dialysis hospitalizations: Trends and outcomes. Stroke. 2014;45:71-6.
- [Google Scholar]
- The three-year incidence of major hemorrhage among older adults initiating chronic dialysis. Canadian Journal of Kidney Health and Disease. 2014;1:21.
- [Google Scholar]
- Clinical features and management of intracranial hemorrhage in patients undergoing maintenance dialysis therapy. Neurol Med Chir (Tokyo). 2004;44:225-32.
- [Google Scholar]
- Subdural hematomas in chronic dialysis patients: Significant and increasing. Clin J Am Soc Nephrol. 2007;2:956-9.
- [Google Scholar]
- Early changes in intracranial pressure during hemofiltration treatment in patients with grade 4 hepatic encephalopathy and acute oliguric renal failure. Nephrol Dial Transplant. 1990;5:192-8.
- [Google Scholar]
- Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodial Int. 2008;12:307-12.
- [Google Scholar]
- Changing the hemodialysis prescription for hemodialysis patients with subdural and intracranial hemorrhage. Hemodial Int. 2013;17(Suppl 1):S22-7.
- [Google Scholar]







