Translate this page into:
Transplant Renal Vein Stenosis (TRVS) Managed with Percutaneous Endovascular Stenting: A Case Report
Corresponding author: Himansu Sekhar Mahapatra, Professor and Head, Department of Nephrology, Dr. Ram Manohar Lohia Hospital, New Delhi, India. E-mail: hsmnephro@gmail.com.
-
Received: ,
Accepted: ,
How to cite this article: Chaudhary P, Tarachandani R, Nath RK, Mahapatra HS. Transplant Renal Vein Stenosis (TRVS) Managed with Percutaneous Endovascular Stenting: A Case Report. Indian J Nephrol. 2025;35:290-2. doi: 10.25259/ijn_193_23
Abstract
Transplant renal vein stenosis (TRVS) is a rare vascular complication of renal transplant that can masquerade findings of rejection and infection. We report a case who presented 2 years 9 months post-transplant with localized non-tender heaviness and fullness at the graft site with renal dysfunction. Initial ultrasonogram (USG) was suggestive of graft pyelonephritis with perinephric collection, though, there were no clinical features of infection and cultures came as sterile. Doppler revealed findings of TRVS, which was confirmed with a CT angiogram. Graft vein angioplasty restored the hemodynamics, but the patient again presented after 4 months with incidentally detected graft dysfunction. USG Doppler showed graft vein stenosis at the same site, which was managed with an elective renal vein angioplasty with stent placement.
Keywords
Transplant renal vein stenosis
Graft vein angioplasty
Renal vein stenting
Graft tenderness
Graft dysfunction
Introduction
Transplant renal vein stenosis (TRVS) is an under-recognized entity.1 Untreated TRVS can result in chronic graft dysfunction progressing to irreversible graft failure.2 Management with endovascular stenting has been reported in very few cases.2,3
Case Report
A 29-year-old male underwent live-related renal allograft transplantation with his wife as the donor. Basiliximab induction followed by standard triple-dose immunosuppression was given. The posttransplant period was uneventful, and immediate graft function was achieved. The patient had stable graft function (baseline serum creatinine 0.6–0.9 mg/dl) for the next 2.5 years.
The first hospital admission was 2 years 9 months posttransplant with the complaintsof heaviness and visible fullness over the graft site. There was no history of pain, tenderness, fever, burning micturition, decrease in urine output, pedal edema, and noncompliance to medications. Urine output was maintained at 1.8–2 l/day. The local examination was nonconclusive with an absence of renal bruit. Relevant investigations are shown in Table 1. Initial graft ultrasonogram (USG) revealed a bulky kidney, suggestive of graft pyelonephritis. Broad-spectrum antimicrobials were started after collecting blood and urine cultures. Serum creatinine gradually increased over the next few days. Repeat graft USG revealed a further increase in graft size. Doppler USG of the graft showed findings of graft renal artery and vein stenosis. Computed tomography (CT) angiography [Figure 1a and b] revealed a normal graft renal artery with narrowing in the renal vein for a length of 15 mm and an ill-defined peripelvic collection (8 × 3 cm) encasing the pelvis, renal vessels, and proximal ureter. USG-guided diagnostic and therapeutic aspiration (70 ml) of the peripelvic collection was suggestive of seroma.
| Parameters | First Admission (2y 9m Post tx) | Second Admission (3y 1m Post tx) | ||
|---|---|---|---|---|
| Pre-Angioplasty | Post-Angioplasty | Pre-Angioplasty | Post-Angioplasty + Stenting | |
| Serum creatinine (mg/dl) | Day 0-1.6 Day 6-1.83 Day 12-1.85 | Day 1-1.1 | Day 0-1.4 | Day 1-1 |
| T0 (ng/ml) | 6.21 | 5.1 | ||
| Urine R/M | Day 0-Pr 3+ RBC 2 | Day 1-Pr 1+ RBC nil D3-NAD | Day 0-Pr 2+ RBC nil | Day 1-Pr 1+ RBC nil Day 2-NAD |
| Spot UPCR | Day 0-2.86 | Day 1-0.16 | Day 0-0.9 | Day 1-0.09 |
| 24-h urine protein (g/day) | Day 0-2.24 | Day 1-0.152 | Day 0-0.809 | Day 1-0.081 |
| Graft size in USG (cm) | Day 0-14 × 5 Day 6-16 × 8 Day 12-18 × 8.3 | Day 1-12.1 × 4.3 | Day 0-14.1 × 5 | Day 1-12.3 × 4.2 |
| Doppler USG | Day 0 | Day 1 | Day 0 | Day 1 |
| Renal artery PSV (cm/s) | 240 | 20 | 142 | 18 |
| Renal artery RI | 0.8 | 0.67 | ||
| Renal vein PSV (cm/s) | 160 | 130 | ||
| Serum | Negative | |||
| Procalcitonin | Sterile | |||
| Blood culture | Sterile | |||
| Urine culture | Sterile | |||
| CMV DNA PCR | Negative | |||
| BKV DNA PCR | Negative | |||
CMV: Cytomegalovirus, PCR: Polymerase chain reaction, PSV: Peak systolic velocity, RI: Resistive index, R/M: Routine microscopy, T0= Trough tacrolimus level, UPCR: Urine protein to creatinine ratio, USG: Ultrasonogram, BKV: BK virus, NAD: No abnormality detected, Pr: Protein, y: Year, m: Month, tx: Transplant
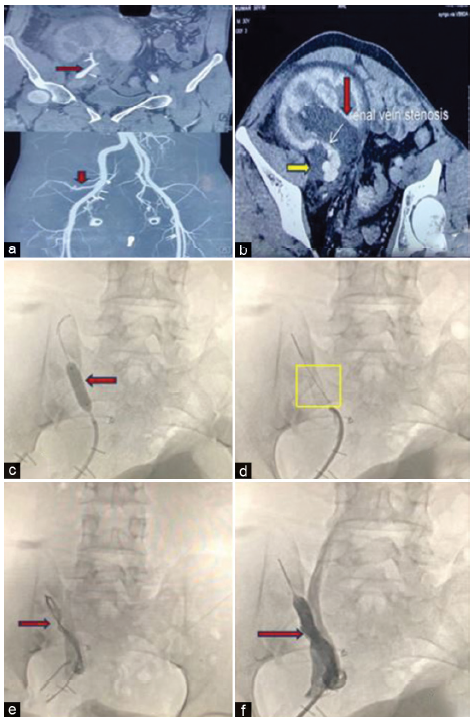
- (a) Normal contrast enhancement of the graft renal artery with no evidence of stenosis. Red arrows show normal contrast enhancement of graft renal artery with no evidence of stenosis (b) Graft renal vein stenosis with hydronephrosis (red arrow) and peripelvic fluid collection (yellow arrow). White arrow shows graft renal vein stenosis. (c) Balloon angioplasty (red arrow) being performed in the stenosed renal vein. (d) Simultaneous renal vein stent placement (yellow square). (e) Compromised flow in the stenosed graft renal vein (red arrow). (f) Significantly improved flow in graft renal vein post-stenting (red arrow).
The patient was planned for digital subtraction angiography with balloon angioplasty with stenting, but had to be taken for an emergency procedure (day 13) with isolated angioplasty because of progressively increasing graft size with new-onset graft tenderness. The procedure was uneventful, and postprocedure graft Doppler USG showed normal flow in the renal graft vein. Oral anticoagulation (rivaroxaban) and low-dose antiplatelet (aspirin 75 mg) were initiated. Repeat blood and urine investigations after the procedure showed normal serum creatinine and negative proteinuria at discharge. Serum creatinine, urine examination, and graft USG Doppler remained normal for the next 3 months postprocedure.
At 4 months postprocedure, the patient was incidentally detected to have graft dysfunction [Table 1]. There was no history of localized heaviness/swelling over the graft, tenderness, burning micturition, and fever. Urine output was adequate (>2 l/day). USG Doppler of the graft was suggestive of an enlarged graft with renal vein restenosis at the previous site, which was confirmed with a repeat CT angiogram. The patient underwent balloon angioplasty with stenting [Figure 1c-f]. Post-stenting graft Doppler parameters improved to normal with normal serum creatinine and urine examination at discharge. The graft function remained stable at subsequent follow-up visits.
Discussion
TRVS is a rare cause of allograft dysfunction, with presentation ranging from weeks to years after transplantation.2 The symptoms and signs are nonspecific and tend to mimic or coincide with graft rejection and local infection. In the present case, the initial presentation was similar to graft pyelonephritis. However, in unexplained graft dysfunction in an enlarged kidney, color Doppler USG remains crucial to diagnosis. TRVS in this case was likely related to external compression from the renal artery crossing above it. Confirming the stenosis also mandates an additional magnetic resonance (MR)/CT angiogram with higher sensitivity and specificity.4
Management of TRVS includes either percutaneous balloon angioplasty with/or without stenting or surgical reconstruction, with the former being a minimally invasive, effective, and safe technique with lower complications.3,5 Percutaneous venoplasty without stent placement is not advocated as it has unsatisfactory long-term outcomes due to early restenosis.6 Restenosis was an expected outcome in the above case, which was subsequently managed with elective renal vein stenting.
TRVS is one of the least suspected etiologies of graft dysfunction, potentially reversible if diagnosed without delay. Doppler USG is an effective screening technique and should be employed early. Balloon angioplasty, along with stent implantation, should be encouraged as the preferred choice of management.
TRVS is one of the least suspected etiologies of graft dysfunction, which is potentially reversible if diagnosed without delay. Doppler USG is an effective screening technique and should be employed early in the presence of a diagnostic dilemma. Balloon angioplasty along with stent implantation should be encouraged as the preferred choice of management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Renal vein stenosis with transudative ascites from graft after renal transplantation with good response after percutaneous stent placement. Transplant Proc. 2014;46:598-601.
- [CrossRef] [PubMed] [Google Scholar]
- Transplant renal vein stenosis: Diagnosis and intervention. J VascInterv Radiol. 2023;34:723-6.
- [CrossRef] [PubMed] [Google Scholar]
- Severe renal vein stenosis of a kidney transplant with beneficial clinical course after successful percutaneous stenting. Am J Transplant. 2008;8:2173-6.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemic nephropathy: More than a simple renal artery arrowing. Iran J Kidney Dis. 2013;7:82-100.
- [PubMed] [Google Scholar]
- Renal vein stenosis after renal transplantation: Treatment with stent placement. J VascInterv Radiol. 2010;21:756-8.
- [CrossRef] [PubMed] [Google Scholar]
- Renal transplant vein stenosis: Demonstration and percutaneous venoplasty of a new vascular complication in the transplant kidney. Clin Radiol. 1991;43:42-6.
- [CrossRef] [PubMed] [Google Scholar]








