Trend of Antimicrobial Resistance of Gram-Negative Uropathogens Post Kidney Transplantation
Corresponding author: Shabna Sulaiman, Department of Nephrology, IQRAA International Hospital and Research Centre, Calicut, India. E-mail: shabnasulaimanss@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sulaiman S, Nair PR, Bhatt AN, Hafeeq B, Uvais NA, Anoop KPM, et al. Trend of Antimicrobial Resistance of Gram-Negative Uropathogens Post Kidney Transplantation. Indian J Nephrol. 2024;34:374-6. doi: 10.25259/IJN_154_2024
Dear Editor,
Urinary tract infections (UTI) are the most common infections seen post kidney transplantation (KT) with antimicrobial resistance (AMR) posing a challenge to graft and patient longevity and adding to healthcare costs.1 AMR surveillance aids in choosing antibiotics,2 reducing treatment costs, and decreasing death rates.1,2 Although cross-sectional studies on the prevalence of AMR among uropathogens amongst kidney transplant recipients are available, longitudinal studies on the trend of AMR, especially to newer antibiotics, are lacking. This study demonstrates the trend of AMR to gram-negative (GN) uropathogens amongst kidney transplant recipients (KTR) for over seven years from 2017 to 2023.
From Jan 2017 to March 2023, 713 KTR were studied, and 198 episodes of bacterial culture-proven UTI occurred in 146 patients (20.5%). Over 98% of causative uropathogens were GN isolates, with Klebsiella pneumoniae (56.9%), E. coli (21.5%), Pseudomonas aeruginosa (10.8%), and Enterobacter spp. (7.2%) being the common uropathogens isolated. The distribution of organisms over the years is shown in Figure S1.
GN isolates displayed universal high resistance to cotrimoxazole (95.5%, 95% CI: 92.7%–98.6%), third-generation cephalosporin (84.6%, 95% CI: 79.6%–89.7%), Ciprofloxacin (74.9%, 95% CI: 68.8%–81.0%), and Cefepime (73.8%, 95% CI: 67.7%–80.0%) [Table S1]. In E. coli, Klebsiella, and Enterobacter, the profile of resistance for nitrofurantoin (p <0.001) and fosfomycin (p = 0.004) were significantly different. Nitrofurantoin and fosfomycin, respectively, showed lower resistance in E. coli (14.3%, 37.5%) compared to Klebsiella (78.4%, 89.2%) and Enterobacter (71.4%, 62.5%). The overall AMR of gram negative bacilli (GNB) to amikacin was 28% [Figure S2], with higher degrees of resistance seen in Enterobacter (61.5%).
Piperacillin-tazobactam, cefoperazone-sulbactam, and meropenem were effective for treating post-transplant UTI with uniformly lower AMR (29.2%, 32.5%, 23%). The resistance patterns of E. coli, Klebsiella spp, Enterobacter spp, and Pseudomonas spp from 2017 to 2023 are shown in Figure 1.
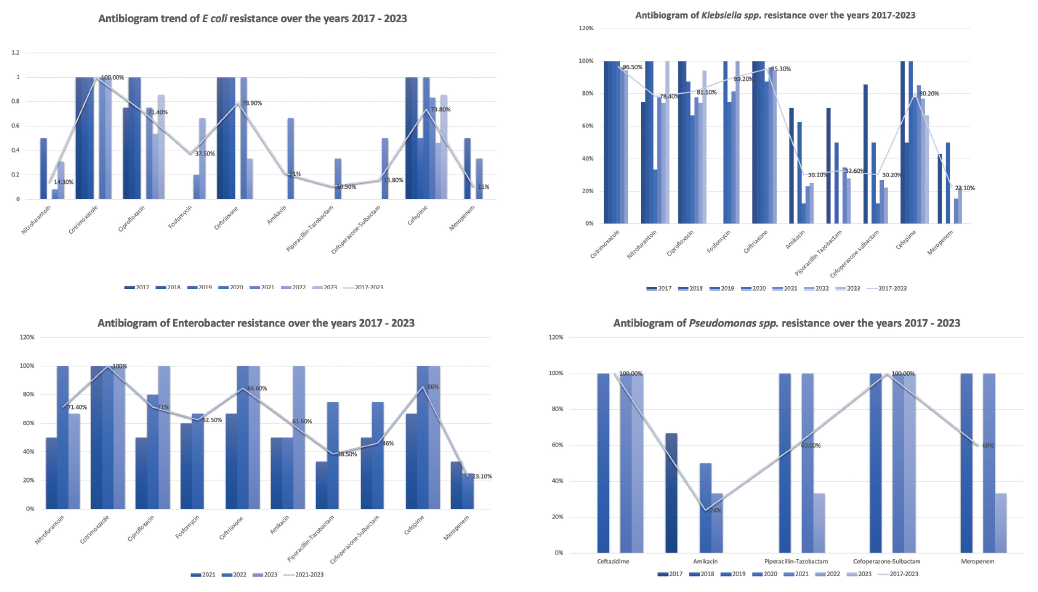
- Organism-wise distribution of AMR from 2017 to 2023.
- *Nitrofurantoin, Cotrimoxazole, and Fosfomycin were not tested against Pseudomonas aeruginosa. AMR: antimicrobial resistance.
Colistin resistance was absent in 14 tested samples, while ceftazidime-avibactam showed resistance in six of seven multidrug-resistant (MDR) isolates, and 4/7 isolates did not show synergy (additive effect with aztreonam). Tigecycline was not considered due to its poor urinary excretion.
Extended-spectrum β-lactamases (ESBL) production was evident in 102 (52.3%) cases, with a significant increasing trend noted since 2021 (p = 0.048). Limited UTI cases precluded trend analysis in earlier years [Figure 2]. Carbapenem resistance was found in 48 (24.6%) cases, without a consistent trend.
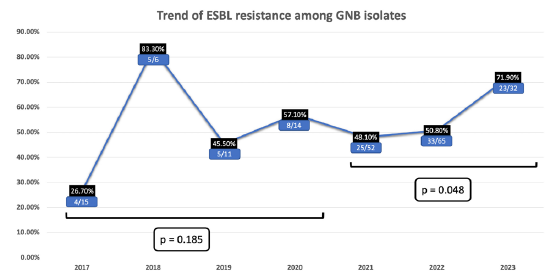
- Incidence of ESBL producing GN uropathogens from 2017 to 2023. p - probability, GN - Gram negative, GNB - Gram negative bacilli, ESBL - Extended-spectrum beta-lactamases
AMR poses a significant challenge in managing post-transplant UTI, imposing a considerable financial burden on healthcare systems globally.3 Addressing this challenge requires ongoing AMR pattern surveillance among uropathogens specific to each region.4 Our seven-year survey in North Kerala, India, addresses this gap, offering insights into clinical implications and guiding empirical antimicrobial therapy decisions. To the best of our knowledge, there is sparse AMR surveillance data for this patient cohort in India. The details of methodology and data analysis are provided in Supplementary Materials and Methods.5
Klebsiella spp. emerged as the leading uropathogen, followed by E. coli, Pseudomonas spp., and Enterobacter spp, contrary to the global trends where E. coli predominates, as shown in the meta-analysis.6 This emphasizes the importance of region-specific surveillance in guiding empirical treatment strategies.
While cotrimoxazole, ciprofloxacin, and third and fourth-generation cephalosporins showed a consistently high degree of AMR, Betalactam-Betalactamase inhibitor (BL-BLI) combinations, carbapenems, aminoglycosides, and fosfomycin exhibited lower resistance rates, aligning with previous studies.S1 Cotrimoxazole, the standard drug used for prophylaxis early post-KT, is also expected to protect against UTI. Based on our results, Cotrimoxazole and fluoroquinolones showed 95.5% and 75% overall AMR, respectively, making it unsuitable to be used as an empirical antibiotic or for UTI antibiotic prophylaxis. Ceftriaxone and aminoglycosides should be avoided for empirical treatment of post-transplant UTI. For suppressive antibiotic prophylaxis, the drugs of choice seem to be fosfomycin or nitrofurantoin.
Despite the global trend of the rising AMR,S1 our study found that overall AMR rates did not significantly change over the study period. The year-wise trend of AMR for various antibiotics has been shown in Figures 1 and S2. The lower incidence of UTI and antibiotic resistance in 2023 could be due to the limited UTI cases recruited only till March 2023.
However, concerning trends emerged, notably the rising incidence of ESBL-producing uropathogens, particularly Klebsiella and E. coli, since 2021. Limited UTI cases in earlier years precluded trend analysis. Korth et al.S1 showed a similar rising trend of ESBL among Klebsiella, but not among E. coli. The incidence of ESBL and carbapenem-resistant organisms among the uropathogens in our study is significantly higher than the reported literature.S2,S3 Previous studies on carbapenem-resistant organismsS4 and ESBLS5 show increased mortality, prolonged hospitalization, and increased economic burden compared to non-MDR infection. This rising incidence of ESBL and carbapenem-resistant organisms indicates that the initial empirical drug regimen should cover ESBL.
Based on the results of the study, we propose a few suggestions to optimize UTI management in KTR—prioritizing culture-guided therapy, tailoring empirical antibiotic regimens based on clinical profiles and institutional antibiograms, and judiciously selecting antibiotics based on susceptibility patterns. Contrary to prior belief, we found that Trimethoprim-sulfamethoxazole was not a good choice in the prophylaxis of GM uropathogens due to increased AMR. Due to the high incidence of ESBL uropathogens in our setting, our protocol was to include Piperacillin-tazobactam, Cefoperazone-sulbactam, and Meropenem as the initial drugs of choice in empirical therapy. Further de-escalation to quinolones, nitrofurantoin, amikacin, or fosfomycin should be guided by culture reports. For extremely drug resistant (XDR) uropathogens, an automated antimicrobial susceptibility testing system should be employed before considering higher antibiotics like colistin, ceftazidime avibactam, and ceftazidime avibactam-aztreonam synergy.
While our study is the first large-scale study to describe the AMR patterns among post-transplant GN uropathogens, it is not without limitations. Interpersonal variations in laboratory practices over the seven years are a limitation. This study is focused on the microbiology and AMR patterns. The clinical and economic impact of post-KT UTI has been described elsewhere (currently under review). Being a single-center study, extrapolation of this data to other centers or different populations requires caution. This study is limited to culture-based results and does not comment on molecular-based detection of AMR.
In conclusion, our study highlights the evolving landscape of post-transplant UTI AMR in North Kerala, advocating tailored interventions to optimize patient outcomes and preserve antimicrobial efficacy.
Acknowledgments
We thank the study participants, their families, and caregivers.
Author’s contributions
SS and PRN had full access to all the data and take full responsibility for the integrity of the data and the accuracy of data analysis. SS and PRN conceptualized, designed, acquired, analyzed, and interpreted the data, as well as drafted the manuscript. Critical revision of the manuscript: All authors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Evaluating the antimicrobial resistance patterns among major bacterial pathogens isolated from clinical specimens taken from patients in mofid children’s hospital, Tehran, Iran: 2013–2018. Infect Drug Resist. 2019;12:2089-102.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antimicrobial resistance and pathogen distribution in hospitalized burn patients: A multicenter study in Southeast China. Medicine (Baltimore). 2018;97:e11977.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibiotic resistance: A rundown of a global crisis. Infect Drug Resist. 2018;11:1645-58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis and management of urinary tract infections in the outpatient setting: A review. JAMA. 2014;312:1677.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence and antimicrobial resistance of bacterial uropathogens isolated from Iranian kidney transplant recipients: A systematic review and meta-analysis. Iran J Public Health. 2019;48:2165-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







