Translate this page into:
Effects of Citrate Acid Concentrate on Hemodialysis Adequacy, Reuse, and Quality of Life: A Prospective Randomized Crossover Trial
Address for correspondence: Dr. S. P. Nagaraju, Department of Nephrology, Kasturba Medical College, Manipal University, OPD No. 15, Manipal, Udupi, Karnataka, India. E-mail: shankarmmcmed@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We conducted a randomized crossover trial to identify whether the use of citrate dialysate (CD) for bicarbonate hemodialysis is beneficial compared to regular acetate dialysate (AD) in terms of adequacy, reuse, and quality of life. Thirty-two stable end-stage renal disease patients on twice-weekly maintenance hemodialysis were randomly assigned to CD or AD fluid in a single-blinded randomized prospective crossover trial of 1-year duration. The primary outcomes studied were the impact of CD in comparison with AD on hemodialysis adequacy, reuse of dialyzer, and quality of life. Secondary outcomes studied were the effect on intradialytic hypotension, acidosis correction, and episodes of symptomatic hypocalcemia. A total number of 28 patients underwent a total of 1456 sessions of hemodialysis with CD over 6 months and 1456 sessions with AD over 6 months. There was a significant increase in dialyzer reuse with the use of CD (P = 0.02). There was no difference in dialyzer adequacy as measured by Single pool Kt/V (spKt/V) (P = 0.840) and urea reduction ratio (%) (P = 0.90). Quality of life did not differ between the two groups. No statistically significant difference was observed in predialysis arterial pH (P = 0.23) serum bicarbonate (0.17) and calcium change (P = 0.16). CD is safe and equally effective as compared to AD. It significantly improves the reuse of dialyzer but it does not offer any added advantage in terms of improvement in hemodialysis adequacy and quality of care.
Keywords
Citrate dialysate
dialysis adequacy
dialyzer reuse
quality of life
Introduction
Citrate dialysate (CD) for bicarbonate hemodialysis has been suggested as biocompatible alternative to acetate-based dialysate (AD). It has been successfully implemented throughout the world for bicarbonate hemodialysis instead of acetate in bicarbonate dialysate.[123] Compared to the traditional AD, which contains 4 mEq/L of acetate; CD has 2.4 mEq/L of citrate and 0.3 mEq/L of acetate. The small amount of citrate used (one-fifth of the concentration adopted in regional anticoagulation) protects against intradialyzer clotting while minimally affecting the calcium concentration. Citrate is a very short-acting anticoagulant (half-life of 49 min) due to its binding with calcium and is quickly metabolized in the liver.[45] Most of the published data in western population showed improved dialysis adequacy, decreased heparin requirements with beneficial effects on systemic hemodynamics.[125678910] No data are available on the effect of use of citrate on quality of life. Effects of CD use on dialysis adequacy and dialyzer reuse in Asian population are lacking. Therefore, the present study was conducted to assess whether use of CD is beneficial compared to AD in terms of adequacy, reuse, and quality of life.
Methodology
After approval from institutional ethics committee with informed consent, 32 stable end-stage renal disease (CKD5D) patients on twice-weekly maintenance hemodialysis of 5 h duration each in our dialysis unit were enrolled in the study. The study was registered with the clinical trials registry - India (CTRI) No. CTRI/2017/05/008546. The inclusion criteria were CKD5D for minimum 3-month duration. Patients with intercurrent illnesses or hospital admissions in the last 1 month, on heparin-free hemodialysis for any reason and irregular for hemodialysis sessions for the last 3 months were excluded from the study.
A single-blind, crossover design was used in which the patients were initially randomized (according to the enrollment sequence) into one of two arms of the study, i.e. assigned to CD or AD fluids. The sample size was calculated based on Cohen's d effect size for reuse of dialyzer between two arms. Assuming moderate effect size of 0.6 between two arms with 80% power and 5% level of significance, the sample size required was 24. Assuming 25% drop out the modified sample size was 32. The study design is as shown in Figure 1. After 6 months, the modality was switched to the alternative one. All patients were dialyzed with arteriovenous fistula as an access with standard blood flow of 300 mL/min and dialysate flow of 500 mL/min. All the patients received standard doses of anticoagulation with unfractionated heparin.

- Single-blind cross over design
The baseline characteristics of all patients were collected as per standard pro forma. Patients were observed for intradialytic hypotension and symptomatic hypocalcemia episodes during the study. Both asymptomatic intradialytic hypotension episodes and symptomatic hypotension episodes requiring nursing intervention as per European Best Practice Guidelines (EBPG) guidelines were observed during the study.[1112] Dialysis adequacy using urea reduction ratio (URR) and single pool Kt/V, acid-base status (prehemodialysis pH and Bicarbonate level), pre- and post-dialysis calcium were assessed during both CD and regular AD dialysate use at the beginning and at the end of 6 months. The reprocessing of the dialyzers was done using automated reprocessing machines and standard protocol was followed as per Indian Society of Nephrology guidelines for hemodialysis units.[13] The fiber bundle volume and pressure leak tests were done at the end of reprocessing as per the protocol and the decision to discard a dialyzer was taken by the nephrologist in charge.[13] Average reuse of dialyzer in each group was analyzed at the end of 6 months. The quality of life was assessed using validated 12-item kidney disease quality of life questionnaire in accordance with the local cultural and language barriers.[14] This quality of life questionnaire domain includes the general health, physical functioning, emotional well-being, sleep, and dialysis symptoms. Before assessing the quality of life in the study patients, the reliability quality of life questionnaire was assessed and it was cronbach's alpha 0.786. The questionnaire was validated and tested for reliability based on the similar study procedure.[141516] The primary outcomes studied were the impact of CD in comparison with AD on hemodialysis adequacy, reuse of dialyzer, and quality of life. The secondary outcomes studied were effect on intradialytic hypotension, acidosis correction, and episodes of symptomatic hypocalcaemia.
The descriptive statistics were computed for baseline characteristics data. That the use of CD is beneficial compared to regular acetate based dialysate in terms of adequacy, reuse, and quality of life was computed by applying independent sample t-test with P < 0.05 considered as statistically significant. The data were analyzed using Statistical Package for the Social Sciences (SPSS) version 15.
Results
Out of 32 patients, 4 patients were withdrawn from the study. Two patients underwent a transplant, one died of acute myocardial infarction and the other moved to another hemodialysis center. Twenty-eight patients underwent a total number of 1456 sessions of hemodialysis with CD over 6 months and 1456 sessions with AD fluid over 6 months (January 2014 to December 2014). Among these patients, 78.5% (n = 22) were males and 25% (n = 7) were diabetics. The mean age of the study patients was 53.46 ± 10.37 years, and the mean duration on dialysis was 3.50 ± 1.77 years. The detailed baseline characteristics data are summarized in Table 1. During the study period, routine medical and clinical practices remained unchanged. Routine dialysis clinical decisions such as changing blood or dialysate flow rates, ultrafiltration during session continued to be made using usual patient/treatment criteria.

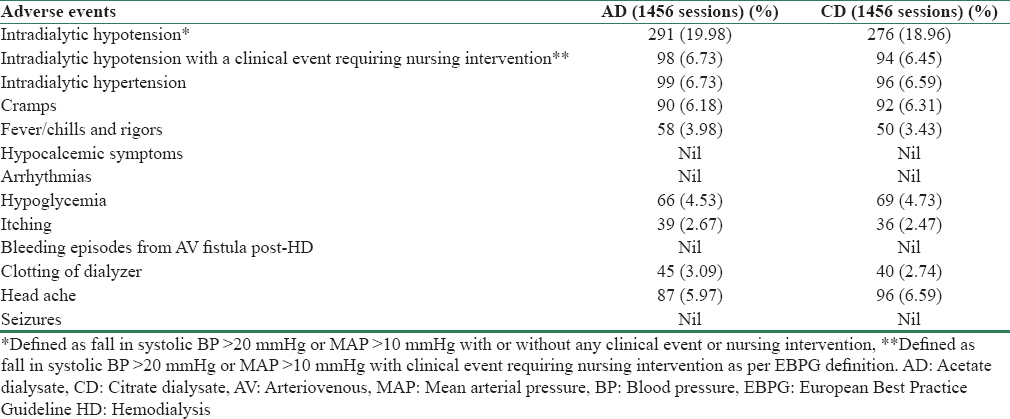
At the end of the study, a significant increase in dialyzer reuse has been observed with CD compared to AD (P = 0.02). No improvement in dialysis efficiency was demonstrated as measured with single pool (spKt/V) and URR (P = 0.84 and 0.90 respectively). Comparison between acid-base changes, adequacy, and reuse between CD and AD are summarized in Table 2. The calcium change (predialysis total calcium to postdialysis calcium) was not significant with the use of citrate (P = 0.16). No statistically significant difference was observed in values of predialysis arterial pH, serum bicarbonate levels (P = 0.23 and 0.17, respectively) [Table 2]. None of the patients developed clinically significant hypocalcemic symptoms and increase in intradialytic hypotension episodes with CD. No significant differences were observed in clotting of dialyzer, bleeding from fistula sites with the use of citrate. The details of adverse events are shown in Table 3. The switch from one dialysate to other was uneventful and made without any adjustments or modification to the machines. No dialysate-related problems occurred throughout the study period.

The quality of life among the CD and AD groups were not significantly different in any of the quality of life domains of general health, physical functioning, emotional well-being, sleep, and dialysis symptoms (P > 0.05). The detailed quality of life scores for CD and AD groups are presented in Table 4.

Discussion
In our study, there was a significant increase in reuse with the use of CD compared to AD (P = 0.02). It is similar to the finding of Ahmad et al. where the overall reuse with CD increased significantly from 15.1 ± 9.4 to 18 ± 10.0 (mean ± standard deviation) on regular AD and CD, respectively (P = 0.0003). It is important in the context of developing countries where reuse is essential for maintaining a cost-effective dialysis.
In the previous studies, it is clear that the use of citrate increased the dialysis efficiency.[16789] Ahmad et al.[1] demonstrated improved dialysis adequacy in a 12-week study of 25 patients with CD. There was a significant URR (68.8 ± 5.9–73.8 ± 5.3%; P < 0.03) and a rise in spKt/V (1.23 ± 0.19–1.34 ± 0.20; P = 0.01). In a study by Kossmann et al.,[7] the use of CD was associated with a significant increase in the delivered dose of dialysis as measured by equilibrated eKt/V urea. He demonstrated improved adequacy in 146 individuals following conversion from standard dialysate to CD. The eKt/V increased from 1.51 ± 0.01 to 1.57 ± 0.01 with CD (P < 0.0001). In our study, there was no improvement in spKt/V with the use of CD (P = 0.84). The URR remained same with the use of AD or CD (P = 0.90). It is probably due to the reason that our patients are on twice-weekly hemodialysis prescription. Perhaps due to the same reason, our patients did not show any improvement in predialysis bicarbonate in contrast to previous studies.[67]
There have been no safety issues reported with CD during the study. In our study, there was no change in pre- and post-HD calcium levels with the use of citrate, and there was no episode of symptomatic hypocalcemia. Nevertheless, we did total serum calcium levels instead of ionized calcium levels pre- and post-HD, which is a limitation. There were no increased episodes of intradialytic hypotension with the use of citrate. We did not study the effect of usage of citrate on decreasing heparin dose like in the previous studies.[8910] However, with the standard use of heparin; there were no bleeding episodes from AV fistula site post-HD reported with the use of citrate in our study.
Ours is the first study to see whether the use of citrate improves quality of life. In our study, we did not find any change in the quality of life with the use of CD for 6 month duration. It is probably due to the short duration of follow-up and the fact that quality of life is affected by multiple factors. The strength of our study is that it is a prospective randomized crossover study of 1-year duration with 6 months on each dialysate. The limitations are that it is a single-center study and we measured total serum calcium levels instead of ionized calcium levels.
Conclusion
Citrate dialysate is safe and equally effective as compared to acetate dialysate. It significantly improves the reuse of dialyzer but it does not offer an added advantage in terms of improvement in hemodialysis adequacy and quality of life.
Financial support and sponsorship
Manipal University
Conflicts of interest
There are no conflicts of interest.
Acknowleadgments
We sincerely thank our Manipal University for research funding this project.
References
- Dialysate made from dry chemicals using citric acid increases dialysis dose. Am J Kidney Dis. 2000;35:493-9.
- [Google Scholar]
- Citrate kinetics in patients receiving long-term hemodialysis therapy. Am J Kidney Dis. 2005;46:903-7.
- [Google Scholar]
- Alternative methods of anticoagulation for dialysis-dependent patients with heparin-induced thrombocytopenia. Semin Dial. 2003;16:61-7.
- [Google Scholar]
- The effect of citrate dialysate on intradialytic heparin dose in haemodialysis patients: Study design of a Randomised controlled trial. BMC Nephrol. 2015;16:147.
- [Google Scholar]
- Citrate-vs.acetate-based dialysate in bicarbonate haemodialysis: Consequences on haemodynamics, coagulation, acid-base status, and electrolytes. BMC Nephrol. 2009;10:7.
- [Google Scholar]
- Increased efficiency of hemodialysis with citrate dialysate: A prospective controlled study. Clin J Am Soc Nephrol. 2009;4:1459-64.
- [Google Scholar]
- Replacement of acetate with citrate in dialysis fluid: A Randomized clinical trial of short term safety and fluid biocompatibility. BMC Nephrol. 2013;14:216.
- [Google Scholar]
- Effects of citrate acid concentrate (citrasate ®) on heparin N requirements and hemodialysis adequacy: A multicenter, prospective noninferiority trial. Blood Purif. 2012;33:199-204.
- [Google Scholar]
- Fifty-five percent heparin reduction is safe with citrate dialysate in chronic dialysis patients. J Am Soc Nephrol. 2006;17:109-10.
- [Google Scholar]
- EBPG guideline on haemodynamic instability. Nephrol Dial Transplant. 2007;22(Suppl 2):ii22-44.
- [Google Scholar]
- Prevalence of intradialytic hypotension, clinical symptoms and nursing interventions – A three-months, prospective study of 3818 haemodialysis sessions. BMC Nephrol. 2016;17:21.
- [Google Scholar]
- Indian society of nephrology guidelines for hemodialysis units. Indian J Nephrol. 2012;22(Suppl 1):S24-6.
- [Google Scholar]
- Cross-cultural adaptation, validation and reliability of the South Indian (Kannada) version of the kidney disease and quality of life (KDQOL-36) instrument. Saudi J Kidney Dis Transpl. 2015;26:1246-52.
- [Google Scholar]
- Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329-38.
- [Google Scholar]
- Kidney Disease Quality of Life Short Form (KDQOL-SFTM). A Manual for Use and Scoring. Ver. 1.3. Santa Monica: RAND; 1997. p. :7994.
- [Google Scholar]







