Translate this page into:
Mesangial C1q Deposition in IgA Nephropathy: Does the Classical Complement Pathway Play an Independent Role?
Corresponding author: Gabriele Gaggero, Pathology Unit, IRCCS Istituto Giannina Gaslini, Largo Gerolamo Gaslini, Genova, Italy. E-mail: gabriele.gaggero@outlook.it
-
Received: ,
Accepted: ,
How to cite this article: Gaggero G, Mazzocco K, Cafferata B, Angeletti A. Mesangial C1q Deposition in IgA Nephropathy: Does the Classical Complement Pathway Play an Independent Role? Indian J Nephrol. 2025;35:116-7. doi: 10.25259/IJN_424_2024
Dear Editor,
We read with great interest the review article by Roberts et al. entitled “IgA nephropathy: Emerging Mechanisms of Disease.”1 This is an in-depth and comprehensive review of the treatment of immunoglobulin A nephropathy (IgAN), focusing on the complex pathological mechanisms underlying IgAN in order to move to increasingly targeted treatments.
We would like to contribute by commenting on an aspect that has not been specifically mentioned, namely the relationship between IgAN and glomerular complement component 1q (C1q) deposition.
A renal biopsy performed in a three-year-old boy presented only for persistent macrohematuria (with no other current or past medical/laboratory history) showed microscopically mesangial hypercellularity with glomerular deposits on immunofluorescence for IgA and C1q [Figure 1]. All other histologic structures, both glomerular (capillaries, basal membrane, podocytes, urinary space, Bowman’s capsule) and non-glomerular (tubules, interstitium, vessels), were negative for any lesions, as were negative immunofluorescence findings for IgG, IgM, C3, and C4d: the diagnosis was IgAN associated with mesangial C1q deposition.
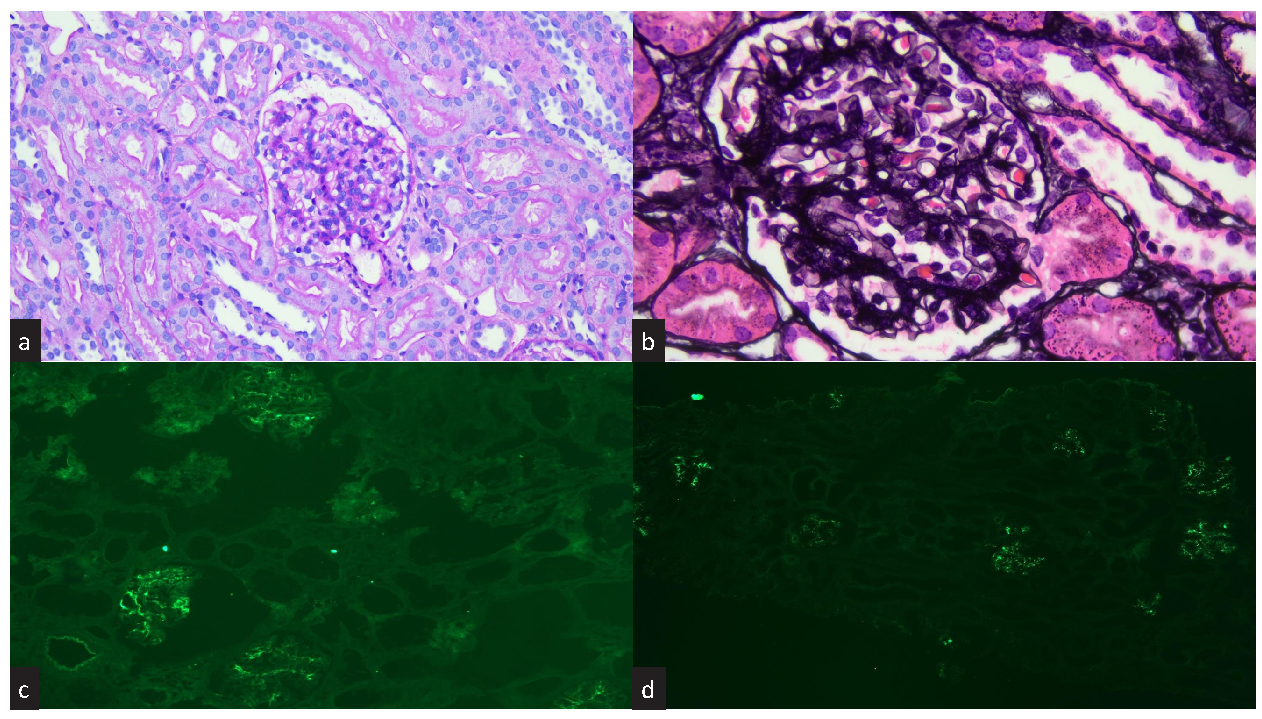
- Histology and immunofluorescence. (a) Photomicrograph showing increased mesangial cellularity and normal endocapillary cellularity (PAS staining, 20x). (b) Photomicrograph showing a slight increase in mesangial reticular texture without true segmental sclerosis (PASM staining, 40x). (c) Positive immunofluorescence for IgA (10x). (d) Positive immunofluorescence for C1q (10x). PASM: Periodic schiff-methenamine silver; PAS: Periodic acid-schiff.
Primary IgAN can progress to end-stage kidney disease in 14–39% of cases.2 Disease progression correlates with the severity of histologic lesions according to the 2016 Oxford Classification: mesangial cellularity (M), endocapillary cellularity (E), segmental glomerulosclerosis (S), tubular atrophy/interstitial fibrosis (T), and crescents (C):3 the described case was classified as M1,S0,T0,C0, highlighting the coexistence of glomerular C1q deposition.
In fact, IgAN/C1q co-occurrence is not so rare, and correlates with a worse outcome in terms of renal survival, in particular with a described correlation with crescent formation, both in native and posttransplant kidneys.4–6
A large part of the review by Roberts et al.1 relies on the article by Suzuki et al., according to which four successive genetic molecular hits are required to reach the actual kidney damage in IgAN. Only the fourth hit, after glomerular deposition of immune complexes with IgA1, leads to complement activation and the resulting inflammatory cascade that increases kidney damage.7 However, both articles focus on the alternative complement pathway, particularly the involvement of C3 and C5, with Roberts et al. stating that disease severity and progression correlates with glomerular C3 deposition and concluding the argument by writing that “the absence of C1q deposition suggests that the activation of the complement cascade does not involve the classical pathway in IgAN,”1 and Suzuki et al. stating that components of the classical complement pathway, including C1q, are typically absent in IgAN.7 This pathogenetic approach is in line with what Zhang et al. write in a very similar recently published paper, referring to “ongoing therapeutic trials investigating inhibitors of components C3 and C5.”8
However, recent data break this axiom and make it less rigid by introducing C1q as a possible building block in IgAN, suggesting both that it plays its own independent role (and we do not yet know whether this is mutually exclusive regarding alternative pathway activation as in our immunofluorescence findings)4–6 and that C1q can therefore be considered in the ever-expanding landscape of IgAN target therapy. Finally, it confirms the role of microscopic examination of kidney biopsies (histopathology and immunofluorescence) not only for the prognostic aspects of the Oxford classification but also to identify cases where pathological glomerular IgA deposition is accompanied by C3 or C1q, thus differentiating patients with distinct potential therapeutic targets.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- IgA Nephropathy: emerging mechanisms of disease. Indian J Nephrol. 2024;34:297-309.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Natural history of primary IgA nephropathy. Semin Nephrol. 2008;28:4-9.
- [CrossRef] [PubMed] [Google Scholar]
- Oxford classification of IgA nephropathy 2016: An update from the IgA nephropathy classification working group. Kidney Int. 2017;91:1014-21.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and prognostic significance of C1q deposition in IgAN patients – a retrospective study. Int Immunopharmacol. 2020;88:106896.
- [CrossRef] [PubMed] [Google Scholar]
- A multicenter, prospective, observational study to determine association of mesangial C1q deposition with renal outcomes in IgA nephropathy. Sci Rep. 2021;11:5467.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and pathological significance of mesangial C1q deposition in kidney transplant recipients with recurrent IgA nephropathy and patients with native IgA nephropathy. Nephron. 2023;147(Suppl 1):80-8.
- [CrossRef] [PubMed] [Google Scholar]
- The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795-803.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Current understanding and new insights in the treatment of IgA nephropathy. Nephrology (Carlton) 2024 (Epub ahead of print)
- [Google Scholar]







