Translate this page into:
Prevalence and Pattern of Upper Gastrointestinal Lesions in Prospective Kidney Transplant Patients in Bangladesh
Corresponding author: Amit Bari, Department of Nephrology, Kidney Foundation Hospital and Research Institute, Dhaka, Bangladesh. E-mail: amit.alimul.bari@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Islam S, Kabir MA, Bari A, Ghosh CK, Alam MR, Khan NT, et al. Prevalence and Pattern of Upper Gastrointestinal Lesions in Prospective Kidney Transplant Patients in Bangladesh. Indian J Nephrol. doi: 10.25259/IJN_66_2024
Abstract
Background
Upper gastrointestinal (GI) lesions are common in patients with chronic kidney disease and may contribute to anemia and increased morbidity, especially in patients on hemodialysis (HD). They become even more significant in patients undergoing kidney transplantation about to be subjected to lifelong immunosuppressive drugs.
Materials and Methods
This cross-sectional study conducted at Bangabandhu Sheikh Mujib Medical University’s Departments of Gastroenterology and Nephrology analyzed 128 prospective kidney transplant patients following upper GI endoscopy. Lesions were histopathologically examined and factors contributing to and consequences of the lesions were investigated.
Results
A total of 52.3% patients had at least one upper GI lesion. Gastritis was the most prevalent one, comprising 55.7% of the lesions, followed by duodenitis (21.6%), ulcer (12.5%), esophagitis (2.3%), and others (8%). Lesions did not vary significantly between symptomatic and asymptomatic patients (p = 0.9). Those on HD had 3.6-fold higher odds of having a lesion (p = 0.005). Patients with lesions had significantly longer CKD duration (p = 0.0002). Mean hemoglobin level was 8.8 g/dl in those with lesions, which was significantly lower compared to those without lesion, who had a mean of 10.3 g/dl (p < 0.0001). Iron deficiency was more common in patients with lesions (p = 0.0004). The mean serum calcium level was significantly higher in patients with lesions (p = 0.0002).
Conclusion
Upper GI lesions are fairly common in Bangladeshi CKD population. Routine endoscopic screening and treatment of asymptomatic lesions are recommended for advanced CKD patients in Bangladesh, given their frequency and potential impact.
Keywords
Chronic kidney disease
Upper gastrointestinal lesions
Renal replacement therapy
Erythropoietin resistant anemia
Gastritis
Introduction
Chronic kidney disease (CKD) is a condition that is increasing in both incidence and prevalence for the past few decades.1 In 2017, there were 697.5 million (9.1% of the total population) cases of CKD worldwide.2 Bangladesh had more than one million estimated cases.3
Patients with CKD have a higher incidence of upper gastrointestinal (GI) lesions, which can deteriorate their quality of life.4–6 Hypergastrinemia, hyperparathyroidism, back diffusion of hydrogen ions, poor cytoprotection, fluctuation in gastric blood supply, higher serum level of urea, and Helicobacter pylori (H. pylori) infection are some of the proposed mechanisms of upper GI lesions in CKD.7,8 Due to decreased kidney function, there is reduced clearance and, consequently higher serum level of urea in CKD. This uremic milieu makes the GI mucosa more vulnerable to trivial injury and leads to increased lesions.9 Also, renal insufficiency in CKD leads to reduced breakdown and clearance of gastrin. The resultant hypergastrinemia leads to increased gastric acid secretion and more upper GI lesions.10
Although CKD patients develop various upper GI symptoms, often there is no demonstrable relationship between symptoms and lesions in this population, making them difficult to diagnose and treat.6 If gone unnoticed, these lesions tend to deteriorate during renal replacement therapies (RRTs). This is especially true for hemodialysis (HD), owing to variable gastric blood supply and regular use of heparin; but may also occur following kidney transplantation, mostly owing to corticosteroids and other high dose immunosuppressive drugs. Therefore, routine upper GI endoscopy is a part of the pretransplant evaluation protocol in most centers.11
Anemia is an important complication of CKD. Often times, it is treated blindly with conventional iron supplements and erythropoietin-stimulating agents (ESAs) without investigating the underlying etiology.12 Upper GI lesions have been considered as an important cause of anemia in the CKD population. Identifying and treating them accurately will lead to a better outcome.12
Our study aimed to investigate upper GI lesions in patients with CKD. As far as our knowledge goes, no prior research has explored the prevalence of upper GI lesions among CKD patients in Bangladesh.
Materials and Methods
This cross-sectional study was carried out in the Department of Gastroenterology and Nephrology of Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, from October 2021 to September 2022. Prospective kidney transplant patients, both on HD and not on dialysis, were selected from the Department of Nephrology, BSMMU. The study excluded patients with chronic liver disease as well as those who had been exposed to nonsteroidal anti-inflammatory drugs (NSAIDs) or other ulcerogenic medications (e.g., proton pump inhibitor, H2 blocker) within the previous four weeks.
Data collection
Baseline demographic data and clinical and biochemical parameters were collected on pretested questionnaires through history, physical examination, and laboratory investigations. Patients with any of the following symptoms at the time of data collection, such as nausea, vomiting, heartburn, abdominal pain, epigastric fullness, or any other symptom perceived by the researcher as originating from the upper GI tract, were considered to have upper GI symptoms. GI endoscopy was done in all patients. Olympus GIF-H190 endoscopy machine using CV-190 processor and CLV-190 light source was used for the endoscopy procedure. In cases where a lesion was identified during upper GI endoscopy, histopathology was performed if indicated. The presence of H. pylori was confirmed through both the rapid urease test and histopathology analysis. Operational definitions of GI lesions are included in the Supplemental Material.
Statistical analysis
All data were systematically recorded on predesigned data collection forms. Descriptive statistics for demographic and clinical characteristics were provided, with quantitative variables reported as mean ± standard deviation or median ± interquartile range (IQR) and qualitative variables presented as frequencies. Statistical analyses were performed by using Statistical Analysis Software (SAS) Studio Version 9.04.01M6P110718. The difference in means or percentages of different variables was calculated using either the chi-square/Fisher’s exact test for nominal variables or the unpaired student t-test for numerical variables. To evaluate the relationships of the different factor associated with the lesions, univariate and multivariate logistic regression models were used where applicable. P-values less than 0.05 were considered significant.
The research study described in this article adheres to ethical guidelines and principles, receiving approval from the Institutional Review Board (IRB) of BSMMU with approval number 3562. Prior to their participation, all patients involved in the study provided informed written consent, ensuring their voluntary participation and protection of their rights and privacy.
Results
The study included 128 patients, with 64.1% being male. Diabetes was present in 22.7% of the patients. Hypertension was the most prevalent comorbidity observed in 94.5% of patients. In advanced CKD cases, it is difficult to determine whether hypertension was the cause or the effect. Table 1 provides additional details on the baseline characteristics of the study population.
| Total (n = 128) | Upper GI lesion (n = 67) | No upper GI lesion (n = 61) | p-value | |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age, yearsa,b | 38.2 (19 – 65) | 35.6 (23 – 65) | 40.3 (19 – 65) | 0.04* |
| Male, n (%)d | 82 (64.1%) | 54 (80.6%) | 28 (45.9%) | <0.0001* |
| Residence, n (%)c | ||||
| Rural | 37 (28.9%) | 10 (14.5%) | 27 (44.3%) | 0.0003* |
| Urban | 89 (69.5%) | 55 (79.7%) | 34 (55.7%) | |
| Semi-urban | 2 (1.6%) | 2 (3%) | 0 | |
| Smoker, n (%)c | ||||
| Current | 8 (6.4%) | 6 (9%) | 2 (3.4%) | 0.52 |
| Former | 42 (33.3%) | 22 (32.8%) | 20 (33.9%) | |
| Non | 76 (60.3%) | 39 (58.2%) | 37 (62.7%) | |
| BMI, n (%)c | ||||
| Normal | 43 (61.4%) | 21 (60%) | 22 (62.9%) | 0.44 |
| Overweight | 14 (20%) | 6 (17.1%) | 8 (22.9%) | |
| Obese | 10 (14.3%) | 5 (14.3%) | 5 (14.3%) | |
| Underweight | 3 (4.3%) | 3 (8.6%) | 0 (0%) | |
| Comorbidities | ||||
| Diabetes, n (%)d | 29 (22.7%) | 14 (20.9%) | 15 (24.6%) | 0.62 |
| Hypertension, n (%)c | 121 (94.5%) | 65 (97%) | 56 (91.8%) | 0.26 |
| Hemodialysis | ||||
| HD patient | 94 (73.4%) | 58 (61.7%) | 36 (38.3) | 0.0004 |
| Non-HD patient | 34 (26.6%) | 25 (73.5%) | 9 (26.5%) | |
| Duration of hemodialysis (months)e | 19.3 (3 – 56) | 25.1 (3 – 56) | 15.8 (4 – 48) | 0.0001 |
| Iron deficiency | ||||
| Present | 67 (52.3%) | 45 (67.2%) | 22 (32.8%) | 0.0004 |
| Absent | 61 (47.7%) | 22 (36.1%) | 39 (63.9%) |
aData is expressed as mean (range), bUnpaired T-test, cFisher’s Exact Test, dChi-Square Test, eData is expressed as mean (SD), *Significant, BMI: Body mass index, CKD: Chronic kidney disease, GI: Gastrointestinal, HD: Hemodialysis.
Out of the 128 patients, 67 (52.3%) had at least one lesion, with some having multiple lesions, resulting in the identification of 88 lesions in total. The most commonly occurring lesions was gastritis (55.7%), followed by duodenitis (21.6%), ulcer (12.5), and esophagitis (2.3). About 5% patients had lesions that were not included in these categories, such as polyps and vascular ectasia. Figure 1 provides a detailed depiction of the findings.
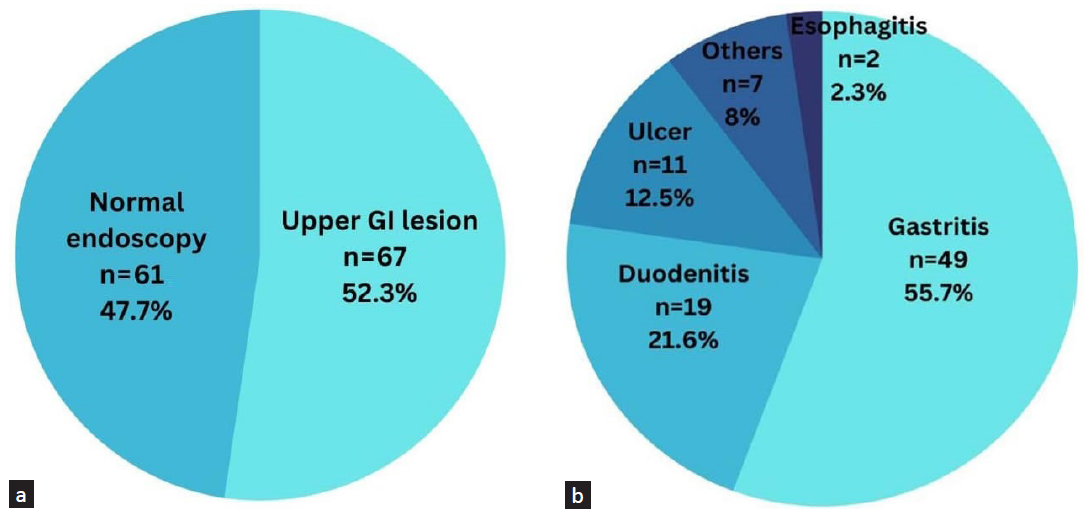
- Pie chart showing endoscopic findings in CKD patients (a = total number of patients with at least one lesion, b = number of individual lesions). GI: gastrointestinal.
Although the H. pylori infection rate appeared to be higher in patients with lesions (46.3%) than those without lesions (32.8%), the difference was not statistically significant (p = 0.1). There was no significant difference in symptoms between the two groups (p = 0.9). Approximately, 41% patients in each group had upper GI symptoms.
Our study found that 61.7% of the 94 patients receiving dialysis had an upper GI lesion, which was significantly higher than patients not on dialysis (p = 0.0004). Among patients receiving dialysis, the duration of dialysis was significantly longer in the group with GI lesions (25.1 months) than in the group without lesions (15.8 months). Gastritis was the most prevalent lesion in both groups, accounting for 46.8% of patients on dialysis. Figure 2 provides further details.
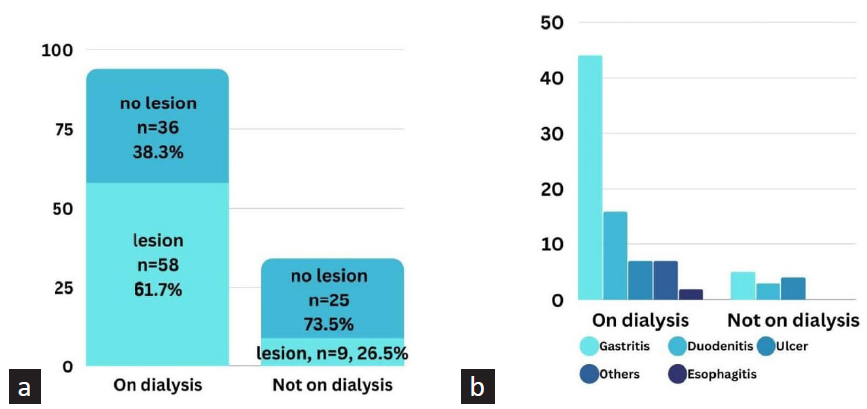
- Bar diagram showing endoscopic findings in CKD patients on and not on dialysis (a = total number of patients with at least one lesion, b = number of individual lesions; Chi-square test showed significantly higher number of lesions in patients on dialysis, p = 0.0004).
The relationship between HD and upper GI lesion was demonstrated through a logistic regression model in Figure 3. After a univariate model showed a positive correlation, a multivariate model was constructed, controlling for H. pylori infection. The multivariate model showed that patients on HD had 3.6-fold increased odds of developing upper GI lesions compared to pre-dialysis patients even after controlling for H. pylori infection, indicating HD to be an important predictor of upper GI lesions in CKD patients. The c-stat was 0.72 in the multivariate model, which improved from 0.64 in the univariate model, suggesting a role of H. pylori and good model fit.
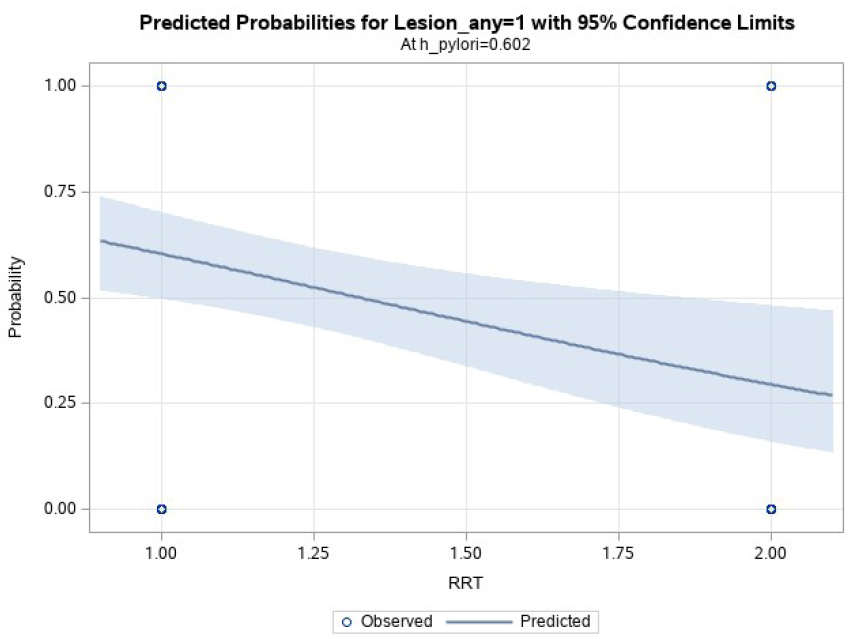
- Multivariate logistic regression model showing the relationship between RRT (hemodialysis) and upper GI lesions after controlling for H. pylori; patients on hemodialysis had 3.6-fold increased risk of developing upper GI lesions compared to pre-dialysis patients; odds ratio = 3.6 (95% CI = 1.5 – 9); p = 0.005. GI: gastrointestinal, RRT: renal replacement therapy.
Patients with upper GI lesions had a significantly higher mean duration of CKD compared to those without lesions, 38.3 (± 17.2) months on the lesion group, and 26.8 (± 17.3) months in the nonlesion group (p = 0.0002).
The mean serum calcium level was significantly higher in patients with lesions (8.8 ± 1 mg/dl) compared to those without lesions (7.8 ± 1.5 mg/dl), with a p-value of 0.0002, as shown in this study.
Patients with lesions had a significantly lower mean hemoglobin level of 8.8 (± 1.7) gm/dl compared to those without lesions, with a mean of 10.3 (± 1.6) gm/dl (p < 0.0001). Iron deficiency was also found to be higher in patients with lesions (67.2%) compared to patients without lesions (32.8%) with a p = 0.0004.
Discussion
In this cross-sectional study, 128 prospective kidney transplant patients were included. Most patients were male and mean age was 38 years. Our patient group was younger compared to the previous studies that looked into upper GI lesions in CKD.5,6,11 Since our patients were undergoing pretransplant evaluation and since the kidney transplant recipients in Bangladesh are usually younger, we had a much younger group than most studies done outside Bangladesh.4,13
Among these 128 CKD patients, 67 (52.3%) were found to have at least one upper GI lesion. The prevalence of upper GI lesion in CKD varied widely in previous studies, ranging from 36% to 68%.4,5,8 Reasons for this wide variation are most likely the use of different definitions of CKD for recruitment, variations in eGFR, methods used for determination of upper GI lesions, interoperator variability, comorbidities, and so on. Despite this wide variation, there seems to be a consensus that CKD population experiences more upper GI lesions compared to the general population. Our results are on par with this observation. Although we did not find any study in Bangladesh that looked at upper GI lesions in CKD, the studies done in the general population presenting with dyspepsia show similar results.14,15
Our study revealed no correlation between GI lesions and symptoms in patients with CKD. This finding is consistent with earlier research that has also reported a weak link between symptoms and upper GI lesions.4 These results emphasize the importance of conducting an endoscopic evaluation of the upper GI mucosa before transplantation. Furthermore, they support the need for regular endoscopic assessments for CKD patients.
We didn’t find any relation between H. pylori infection and upper GI lesions. Past studies had mixed results regarding this issue. Few found no relation, while few found H. pylori to be higher in lesions.8,16 Our result suggests that factors other than H. pylori are playing a role in developing upper GI lesions in CKD patients.
HD is associated with a 3.6-fold increase in the likelihood of developing upper GI lesions compared to pre-dialysis patients, even after controlling for H. pylori infection. This contradicts some previous studies, which found higher lesions in the earlier stages of CKD.5,8,17 The pattern of lesions in the two groups differed, with gastritis being predominant in the dialysis group and the non-dialysis group having fewer gastritis and more ulcers. Possible explanation for the discrepancy between our study and previous ones could be that our study mostly had CKD stages 4 and 5 and ESKD patients, whereas those studies included all stages of CKD. Glomerulonephritis, a leading cause of CKD, may have exposed patients in earlier stages to high doses of immunosuppressive drugs, increasing the risk of upper GI lesions. In contrast, most of our patients had been on HD for a longer period. Prolonged exposure to fluctuating blood supply and eventual ischemic damage could account for the increased prevalence of lesions in the dialysis group.4,10 If we consider our immunosuppressive-induced upper GI lesion theory, it is possible that pre-dialytic patients had more ulcers or less healing of ulcers due to drugs. While in the dialysis group, owing to the hypoxic theory, they experienced more trivial lesions in larger numbers.
Patients with upper GI lesions had a significantly longer duration of CKD compared to those without lesions, but this is subject to recall bias due to lack of medical record-keeping in Bangladesh. Previous studies showed varying mean durations of CKD and no difference in lesion frequency or pattern based on duration.6,11
We found a significant association between higher serum calcium levels and the presence of upper GI lesions in CKD patients. CKD mineral bone disease is a known complication of CKD and patients typically receive treatment with phosphate binders, calcium, and vitamin D supplements. However, overtreatment can lead to adynamic bone disease, which has been on the rise in recent years.18 This study did not include a full calcium panel, but it is possible that the higher serum calcium levels in our patients, possibly due to low albumin, contributed to the development of upper GI lesions along with high parathyroid hormone levels.
The lesion group had a significantly lower mean hemoglobin level, and iron deficiency was significantly associated with the presence of upper GI lesions according to an iron profile evaluation conducted in this study. Few previous studies also reported similar findings.13,19 Anemia is a common complication of CKD, and proper evaluation is necessary as it is often multifactorial. Obscure upper GI bleeding is a contributing factor and can be mistaken for erythropoietin-resistant anemia or iron deficient anemia due to the poor absorption of iron. This study highlights the importance of a thorough GI evaluation for difficult-to-manage anemia in CKD.
Our study investigates the prevalence and pattern of upper GI lesions in Bangladeshi CKD population and factors associated with them. Future prospective studies should look at the timeline of development of these lesions and their true impact on the development of anemia. To our knowledge, this is the first of its kind to have looked at upper GI mucosa in CKD patients in Bangladesh. This study has its limitations. Being a cross-sectional study, we could not follow up with the patients and see the true effect of treatment on the evolution of anemia and CKD-(mineral and bone disorder) MBD and their relationship with upper GI lesions. Absence of past medical records of these patients, the role of immunosuppressive and other drugs in the development of the lesions could not be properly evaluated. Physician’s impression had to be relied on in most cases for the determination of the cause of CKD, as renal biopsy was rarely performed and tissue diagnosis was not available in most instances. The single-center design and lack of CKD patients of earlier stages and on other RRT modality make it less generalizable.
Upper GI lesions are highly prevalent in CKD patients, gastritis being the commonest. They are not necessarily related to symptoms or H. pylori infection. Longer duration of CKD, being on HD and elevated serum calcium levels were related to higher frequencies of lesions. Anemia and iron deficiency were more common in patients with upper GI lesions. Routine endoscopy should be considered in CKD patients, especially when managing difficult-to-control anemia.
Conflicts of interest
There are no conflicts of interest.
References
- National trends in the prevalence of chronic kidney disease among racial/ethnic and socioeconomic status groups, 1988–2016. JAMA Netw Open. 2020;3:e207932.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709-33.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of chronic kidney disease in Bangladesh: A systematic review and meta-analysis. Int Urol Nephrol. 2021;53:713-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Upper gastrointestinal manifestations in chronic renal failure through upper gastrointestinal endoscopy. India: International Journal of Scientific Study; 2017.
- Endoscopic findings in end-stage renal disease. Endoscopy. 2003;35:502-5.
- [CrossRef] [PubMed] [Google Scholar]
- Upper gastrointestinal mucosa in chronic renal failure – An endoscopic and histological evaluation. Med J Armed Forces India. 1999;55:307-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Pro. 2007;39:1003-7.
- [CrossRef] [Google Scholar]
- Uremic and post-transplant gastropathy in patients with chronic kidney disease and end-stage renal disease. Cureus. 2020;12:e10578.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gastrointestinal and hepatic disorders in end-stage renal disease and renal transplant recipients. Adv Ren Replace Ther. 2000;7:220-30.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic findings in hemodialysis patients upon workup for kidney transplantation. Saudi J Kidney Dis Transpl. 2020;31:388-94.
- [CrossRef] [PubMed] [Google Scholar]
- Anemia in chronic kidney disease. Pediatr Nephrol. 2018;33:227-38.
- [CrossRef] [PubMed] [Google Scholar]
- Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic and histopathological findings in adult dyspeptic patients, and their association with. IJID Reg. 2022;2:30-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Helicobacter pylori infection and endoscopic findings in Bangladeshi dyspeptic patients. Mymensingh Med J. 2022;31:161-4.
- [PubMed] [Google Scholar]
- Risk factors for peptic ulcer disease in patients with end-stage renal disease receiving dialysis. Kidney Res Clin Pract. 2019;38:81-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for peptic ulcer disease in renal transplant patients—11 years of experience from a single center. Clin Nephrol. 2004;62:14-20.
- [PubMed] [Google Scholar]
- Chronic kidney disease-mineral and bone disorder (CKD-MBD): Current perspectives. Int J Nephrol Renovasc Dis. 2019;12:263-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gastrointestinal lesions in chronic kidney disease patients with anaemia. Nefrologia. 2019;39:50-7.
- [CrossRef] [PubMed] [Google Scholar]








