Translate this page into:
Role of Intravenous Ascorbic Acid in the Management of Anemia in Hemodialysis Patients
Address for correspondence: Dr. Ahmad Alghitany, Department of Nephrology, Faculty of Medicine, Ain Shams University, Abbasia square, Cairo, Egypt. E-mail: ahmedghitany@yahoo.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Patients with end-stage kidney disease (ESKD) suffer from functional iron deficiency where despite the presence of sufficient iron stores in the body, adequate iron is unavailable for heme synthesis. This study hypothesis was that in patients undergoing hemodialysis (HD), administration of intravenous (IV) ascorbic acid (AA) exerts a good effect on the management of anemia, either by increasing the mobilization of iron from tissue stores or acting as an antioxidant to overcome the inflammatory block and increase the erythropoietin sensitivity.
Methods:
Fifty patients with ESRD who were on regular HD were included in the study. Patients' ferritin levels ranged from 500 to 1200 ng/mL with transferrin saturation of 30% or more. However, all patients were anemic and received erythropoietin therapy. Iron therapy was discontinued in the first group, whereas it was continued in the second group that received IV AA.
Results:
A significant increase in the levels of Hb was observed in the second group after 6 months despite the decrease in ferritin levels in both the groups. Transferrin saturation decreased in both groups, the decrease being more in the first group. The levels of C-reactive protein (CRP) decreased in the second group, whereas these increased in the first group.
Conclusions:
Intravenous AA as an adjuvant therapy with iron exerts a favorable and significant effect on the Hb, serum ferritin, and CRP levels in patients with ESKD having anemia. The discontinuation of iron therapy only decreases the serum ferritin levels and does not improve the Hb or CRP levels.
Keywords
Anemia
ascorbic acid
end-stage renal disease
Introduction
The National Kidney Foundation (NKF) defines anemia as a state where the hemoglobin (Hb) concentration is <12 g/dL for women and <13.5 g/dL for men.[1] Anemia is considered as one of the most common complications of end-stage kidney disease (ESKD) and is associated with poor outcomes such as fatigue and shortness of breath.[2] According to a study, the prevalence of anemia in patients with chronic kidney disease (CKD) is double than its prevalence in the normal population. Moreover, anemia may affect up to half of the patients undergoing hemodialysis (HD).[3]
Anemia in patients with ESKD patients is linked to several complications such as heart failure, left ventricular hypertrophy, impaired cognitive and mental functions, and impaired immunity.[4] The most common cause of resistance of patients to erythropoietin is absolute or functional iron deficiency anemia (FID). Other causes include chronic inflammation and infection, folate and vitamin B12 deficiencies, hyperparathyroidism, aluminum toxicity, and the use of angiotensin- converting enzyme inhibitor.[5] Absolute iron deficiency refers to the absence of iron stores in the body, whereas the condition of decreased accessibility of iron stores for physiological functions is referred to as functional iron deficiency (FID) anemia.[6] FID is of two types: type one may occur when erythropoiesis is stimulated pharmacologically by erythropoiesis stimulating agents (ESA) therapy with limited iron supplement, and type two occurs when there is an inflammatory block of iron release from its stores in the reticuloendothelial system.[7]
Iron is recycled following the uptake of red blood cells by reticuloendothelial macrophages. The iron content of these cells could be used for hematopoiesis or could be stored for further use. Iron is mainly regulated by a hormone called hepcidin, secreted by the liver. Hepcidin binds to iron transporter ferroportin, located in erythrocyte membrane, thereby preventing the transport of iron. Thus, iron is not absorbed or recycled by phagocytosis in the presence of hepcidin, resulting in the reduced levels of circulating iron.[8] Vitamin C, present in ascorbic acid (AA) acts as an antioxidant; it is known to enhance the intestinal absorption of iron. Moreover, it aids in the release of iron from ferritin thus mobilizing it from the reticuloendothelial system, resulting in its increased utilization in erythropoiesis. Studies have been conducted to evaluate the effect of intravenous (IV) AA for treating patients on HD with anemia.[9]
The administration of intravenous AA to patients on HD supposed to improve outcomes and better management of anemia. In HD patients, AA has an influence on improving sensitivity to erythropoietin (EPO), either by increasing iron mobilization from tissue storage or by way of antioxidant effects.[1011] Also, it was found that the acute administration of vitamin C reduced oxidant stress levels and improved NO-mediated resistance vessel dilatation in renal failure.[12] On the contrary, other studies found a poor response even with higher and lower doses of IV AA, which were administrated for 3 months. So, the effect of AA on iron indices and ferritin concentration in different trials was variable.[91314]
In the present study, we assessed the effect of intravenous AA on the management of anemia in patients with ESKD and high ferritin levels.
Methods
Patients with ESKD who were undergoing regular HD in the Egyptian Railway Medical Center were enrolled in the study. The experimental procedures were conducted in accordance with the ethical standards of the committee on human experimentation of Ain Shams University and approved by the ethical committee. Written informed consent was obtained from patients before randomization and data collection. There were 36 males and 14 females whose ages ranged between 38 and 82 years. Patients were randomly divided into two equal groups. All the patients were adequately dialyzed with Kt/V over 1.2, and all were doing dialysis three times weekly. All of them had been on HD for more than 6 months and had been receiving erythropoietin as darbepoetin alfa at a dosage of 1.5 mg/kg/wk. Their Hb levels were less than 11.0 g/dL and ferritin levels were in the range of 500–1200 ng/mL. The transferrin saturation was more than 30%. The patients in the first group were discontinued from taking any iron supplement. Those in the second group were administered their maintenance dose of IV iron supplementation as 100 mg iron sucrose once weekly with adjuvant therapy of 500 mg of IV AA after each dialysis session, thrice a week, during the first week of each month (a total of 1500 mg/month). The duration of the study was 6 months. The patients with bone marrow malignancy, myelodysplastic syndrome, hemochromatosis, hemoglobinopathies, any active infection, or blood transfusion during the past 6 months were excluded from the study. All patients consented to be enrolled into the study. The medical history of patients was obtained, and clinical examination was performed. All laboratory investigations were done at baseline, and after 3 and 6 months. Blood samples were collected before the mid-week session for laboratory investigations, including serum creatinine, urea, albumin, iron, ferritin, total iron-binding capacity (TIBC), transferrin saturation, total calcium, phosphorus, parathyroid hormone, complete blood count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP).
Statistical analysis
Data were analyzed using version 21 of the statistical package SPSS. Mean and standard deviation [of quantitative variables and frequencies (number of cases) and relative frequencies (percentages)], were used to summarize the categorical data. The medians and interquartile ranges of the numerical data are presented. Comparisons were performed using the nonparametrical Mann–Whitney U test. Chi square (χ2) test was used for comparing categorical data. When the expected frequency is less than 5, exact the test was used instead. Correlations between quantitative variables were obtained using the Spearman correlation coefficient. The P values were considered statistically significant if less than 0.05.
Results
The present study included 50 patients with ESKD undergoing HD who were randomly divided into two equal groups. The demography and baseline values of laboratory investigations are shown in Table 1. Baseline, 3 month and 6th months values of various variables are shown in Table 2 and Figure [1,2,3].
| Groups | P | ||
|---|---|---|---|
| Group 1 | Group 2 | ||
| Age (Years) | 58.84±6.39 | 58.16±8.41 | 0.75 |
| Duration of dialysis (months) | 26.56±18.6 | 31.04±19.17 | 0.41 |
| Gender | |||
| Female | 5 (20%) | 9 (36%) | 0.21 |
| Male | 20 (80%) | 16 (64%) | |
| Diabetes mellitus (DM) | 11 (44%) | 8 (32%) | 0.382 |
| Hypertension (HTN) | 22 (88%) | 23 (92%) | 1 |
| Albumin (mg/dL) | 3.85±0.26 | 3.81±0.33 | 0.67 |
| Parathyroid hormone (PTH) (pg/mL) | 254.32±170.65 | 247.04±170.96 | 0.88 |
| ESR (mm/h) | 56±15.78 | 53.56±20.45 | 0.64 |
| Baseline Hb (g/L) | 9.47±1.23 | 9.14±0.84 | 0.27 |
| Baseline CRP (mg/L) | 8.84±2.3 | 8.6±2.90 | 0.75 |
| Baseline Ferritin (ng/mL) | 796.87±156.44 | 822±182.59 | 0.6 |
| Baseline Iron (µg/dL) | 80.48±±19.41 | 108.52±17.13 | <0.001 |
| Baseline TIBC (µg/dL) | 205.34±29.87 | 241.8±±33.28 | <0.001 |
| Baseline TSAT (%) | 40±7 | 45±5 | 0.4 |
| Groups | Baseline | 3 months | 6 months | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Hb (g/L) | Group (1) | 9.47 | 1.23 | 9.55 | 1.29 | 9.83 | 1.15 | 0.006 |
| Group (2) | 9.14 | 0.84 | 9.76 | 0.79 | 10.2 | 0.89 | ||
| Ferritin (ng/mL) | Group (1) | 796.87 | 156.44 | 597.2 | 144.62 | 521.68 | 144.23 | 0.374 |
| Group (2) | 822 | 182.59 | 645.87 | 164.95 | 582.36 | 158.7 | ||
| Iron (µg/dL) | Group (1) | 80.48 | 19.41 | 65.44 | 17.3 | 55.64 | 17.21 | 0.043 |
| Group (2) | 108.52 | 17.13 | 99 | 14.98 | 90.96 | 14.39 | ||
| TIBC (µg/dL) | Group (1) | 205.34 | 29.87 | 210.02 | 37.38 | 212.08 | 34.65 | <0.001 |
| Group (2) | 241.8 | 33.28 | 210.62 | 27.15 | 186.36 | 24.01 | ||
| TSAT (%) | Group (1) | 40 | 7 | 31 | 7 | 26 | 7 | <0.001 |
| Group (2) | 45 | 5 | 47 | 5 | 49 | 5 | ||
| CRP (mg/L) | Group (1) | 8.84 | 2.3 | 9.05 | 2.45 | 9.85 | 2.16 | <0.001 |
| Group (2) | 8.6 | 2.9 | 6.98 | 2.49 | 6.21 | 2.47 | ||

- Effect of vitamin C as adjuvant therapy versus discontinuing iron supplement on hemoglobin
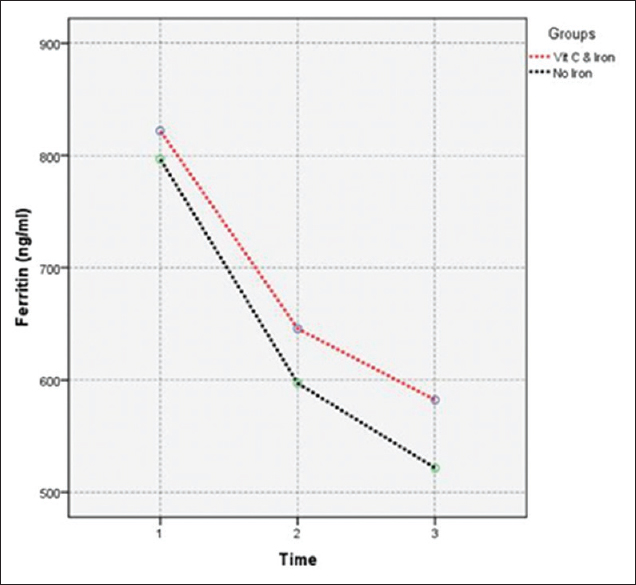
- Effect of vitamin C as adjuvant therapy versus discontinuing iron supplement on serum ferritin levels
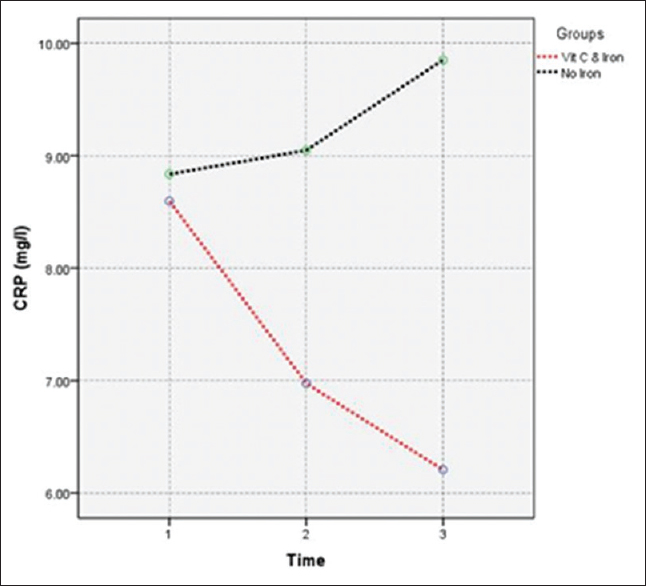
- Effect of vitamin C as adjuvant therapy versus discontinuing iron supplement on CRP
Discussion
Functional iron deficiency is commonly observed in patients with kidney diseases, especially those undergoing HD, where iron moves from the circulation into the storage tissues, making it unavailable for erythropoiesis.
Although it is recognized that FID might be present in patients with HD having high ferritin levels, no practical guidance is available for iron management in patients most likely to have FID. This is because not much progress has been made in strategies to measure iron status in these patients and the assessment of serum ferritin levels continues to be the focus of attention.
One of the therapies recommended to patients with CKD having anemia is iron supplementation. Patients with renal failure can be categorized as having absolute iron deficiency where ferritin is low and transferrin saturation (TSAT) is less than 20% or FID where ferritin is normal or high while TSAT is low. Our patients had neither of these conditions and received adequate erythropoietin supplementation. AA is a rich source of vitamin C. Therefore, we used IV AA to increase the responsiveness to erythropoietin received.[15] The current study assessed administering these patients an adjuvant therapy of IV AA with a maintenance dose of iron therapy, on Hb, serum ferritin, and CRP levels, and iron profile.
The purpose of not providing iron therapy to group 1 was to eliminate its effect on inflammation; moreover, the iron status was satisfactory in this group to continue with the erythropoietin therapy. Interestingly, despite being iron saturated and treated with erythropoietin, we did not observe a rise in Hb levels and the levels of inflammation markers were considerably high. The other group, in addition to receiving the iron and erythropoietin therapy, was administered AA, which we believe contributed to reducing the inflammation and increasing the Hb level. The presence of AA enhanced the utilization of iron in patients in group 2 (absent in group 1), with a consequent reduction in inflammation.
Iron is essential for heme synthesis, and adequate amounts of it are required for the manufacture of new red blood cells. Thus, more iron is used under enhanced erythropoietic stimulation, particularly in those on HD who have inadequate amounts of available iron to satisfy the increased demands of the bone marrow. The discontinuation of iron supplement in group 1 led to decreased iron availability for erythropoiesis and decreased iron stores, reflected by decreased serum ferritin levels and increased TIBC. Hence, patients in group 1 did not show a significant increase in Hb levels. This finding is supported by that of Macdougall who postulated that patients with HD often require IV iron supplementation to maintain iron stores and replace the iron loss.[16]
In contrast, group 2 reported a significant increase in Hb levels, with improved iron status, reflected by decreased serum iron levels, decreased total iron-binding capacity (TIBC), increased TSAT, and decreased serum ferritin levels. These results could be explained by the fact that patients in the current study had a functional block in iron incorporation during heme synthesis, reflected by low Hb levels associated with high levels of iron stores (high serum ferritin levels).
Group 2 has a chronic inflammatory status reflected by high levels of CRP and serum ferritin. This finding is supported by the Kidney Disease Improving Global Outcomes (KDIGO) that postulated that in the absence of a clinically evident infection or inflammatory process, CRP could be used to detect a hidden inflammatory state that may be associated with a high ferritin level and ESA resistance.[17] Results of group 2 suggest that IV AA treatment aided the mobilization of stored iron in these patients. Vitamin C in AA promotes the release of iron from ferritin, thereby mobilizing it from the reticuloendothelial system for use in erythropoiesis.[18] Resistant anemia in our patients could be attributed to the chronic inflammatory status of HD patients although this group cannot be described as FID as TSAT was normal, but still chronic inflammatory state could affect responsiveness to erythropoietin supplementation.
Findings of the current study are similar to that of Attallah et al. who demonstrated that patients with refractory anemia and hyperferritinemia undergoing HD, vitamin C improved the responsiveness to EPO, either by augmenting iron mobilization from tissue stores or through antioxidant effects.[19] Furthermore, the antioxidant effect of vitamin C is reflected by decreased levels of inflammatory markers, supported by decreased CRP and serum ferritin levels in group 2, whereas group 1 showed increased levels of CRP.
One of the major limitations of this study is, single centered with small sample size and short duration period of follow-up. Hence, further studies with large sample size are warranted.
Conclusions
Intravenous AA as adjuvant therapy with iron has a favorable and significant effect on increasing the Hb levels and decreasing serum ferritin and CRP levels. The discontinuation of iron therapy decreases the serum ferritin levels; however, it does not improve Hb levels or the chronic inflammatory status.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Am J Kidney Dis. 2006;47:S1-S108.
- Prevalence of CKD in the United States: A sensitivity analysis using the national health and nutrition examination survey (NHANES) 1999–2004. Am J Kidney Dis. 2009;53:218-28.
- [Google Scholar]
- Efficacy of oral iron therapy in patients receiving recombinant human erythropoietin. Am J Kidney Dis. 1995;25:433-9.
- [Google Scholar]
- Pharmacologic adjuvants to epoetin in the treatment of anemia in patients on hemodialysis. HemodialInt. 2005;9:7-22.
- [Google Scholar]
- Intravenous ascorbic acid in hemodialysis patients with functional iron deficiency. Nephrol Dial Transplant. 2000;15:1717-8.
- [Google Scholar]
- Intravenous iron preparations and ascorbic acid: Effects on chelatable and bioavailable iron. Kidney Int. 2005;67:1161-70.
- [Google Scholar]
- Effect of short-term intravenous ascorbic acid on reducing ferritin in hemodialysis patients. Indian J Nephrol. 2012;22:168-73.
- [Google Scholar]
- Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int. 2003;63:1433-42.
- [Google Scholar]
- Comparison of intravenous ascorbic acid versus intravenous iron for functional iron deficiency in hemodialysis patients. Nippon Jinzo Gakkai Shi. 2004;46:804-9.
- [Google Scholar]
- Effect of vitamin C supplementation on C-reactive protein levels in patients undergoing hemodialysis: A randomized, double blind, placebo-controlled study. Nephrourol Mon. 2013;6:e13351.
- [Google Scholar]
- Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1:S4-8.
- [Google Scholar]
- Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: Oral or intravenous? Curr Med Res Opin. 2010;26:473-82.
- [Google Scholar]
- Kidney Int Suppl. 2012;2:292-8.
- Vitamin C neglect in hemodialysis: Sailing between Scylla and Charybdis. Blood Purif. 2007;25:58-61.
- [Google Scholar]
- Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. Am J Kidney Dis. 2006;47:644-54.
- [Google Scholar]







