Sono-graphic Rim Sign in Postpartum Renal Cortical Necrosis: Experience at a Tertiary Care Centre
Corresponding author: Adarsh Kumar, Department of Nephrology, VMMC & Safdarjung Hospital, Ansari Nagar East, New Delhi, Delhi, India. E-mail: adarshnephro081@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar A, Rajput M, Kumar R, Mahajan S, Jain C. Sono-graphic Rim Sign in Postpartum Renal Cortical Necrosis: Experience at a Tertiary Care Centre. Indian J Nephrol. doi: 10.25259/IJN_244_2024
Abstract
Background:
Kidney biopsy or contrast studies are required to diagnose acute renal cortical necrosis (RCN). Both procedures may be potentially delayed in the postpartum setting. Contrast-enhanced ultrasound (CEUS) is a potential alternative for these patients but remains limited in availability. Due to sparse literature and the rarity of RCN, the role of conventional B-mode ultrasound (USG) in diagnosing RCN remains unexplored.
Materials and Methods:
This retrospective study involved postpartum patients with RCN who underwent kidney biopsy at a tertiary care center. Medical records and B-mode USG kidney images of all 23 patients with postpartum RCN over the past 24 months were retrieved. Gray-scale B-mode USG images of the kidney were assessed for size, presence of sonographic hypoechoic rim, USG kidney timing, echogenicity, and cortico-medullary differentiation.
Results:
Among the 23 patients, sonographic hypoechoic rim was observed in 6 patients (26.1%). USG kidney timing ranged from 1.1 to 8 weeks, and kidney length varied from 9.0 to 10.8 cm. There were significant differences in mean USG kidney timing and mean kidney length between patients with and without hypoechoic rim (P= 0.020 and P= 0.036, respectively). The mean USG kidney timing was notably earlier in patients with sonographic rim signs than those without sonographic rim signs (2.5 ± 0.77 weeks and 4.81 ± 2.17 weeks, respectively).
Conclusion:
Despite its lower sensitivity, the sonographic rim sign is an important diagnostic feature of postpartum renal cortical necrosis. In point-of-care ultrasonography, it should be looked for early in the disease course, especially in cases of postpartum anuric acute kidney injury.
Keywords
Postpartum renal cortical necrosis
Sono-graphic rim sign
Pregnancy-related acute kidney injury
Introduction
Acute renal cortical necrosis (RCN) is an uncommon cause of renal failure, often resulting in severe renal outcome. RCN is frequently associated with complications related to pregnancy, severe shock, certain toxins (such as snake venom and drugs), acute pancreatitis, complications of renal transplant, and severe sepsis. Histopathologically, RCN manifests as necrosis of the glomeruli and tubules, with fibrin thrombi noted in the renal capillaries as a pathologic correlate.1 Fogo et al. visualized the coagulative necrosis of the cortex with relative sparing of the medulla.2 Because of tertiary care center with referrals from adjoining states, our hospital receives a large number of patients with postpartum acute kidney injury (AKI) requiring dialysis. Diagnosis of RCN typically relies on contrast studies or renal biopsy. However, these investigations are often avoided postpartum due to nephrotoxic insult and bleeding risks. While contrast-enhanced ultrasound (CEUS) has emerged as a potential diagnostic tool, its availability remains limited. Conventional B-mode USG is readily available and often performed at the bedside for AKI patients. Literature on early sonographic features of RCN is scarce. A hypoechoic renal cortex on USG, aptly called “sonographic hypo-echoic cortical rim,” is a diagnostic feature of RCN. Point-of-care ultrasonography (POCUS) in the hands of a nephrologist has become a useful diagnostic tool.3 This study was undertaken to analyze the USG finding of the hypoechoic renal cortex in biopsy-proven cases of postpartum RCN.
Materials and Methods
This retrospective study included cases of biopsy-proven postpartum RCN patients who underwent kidney biopsy at our hospital from January 2022 to December 2023. This study was registered with the local ethical committee of our institution. All the study data adheres to the 2013 amended Declaration of Helsinki principles. USG images were anonymized to avoid their affiliation with the patient’s identity. Inclusion criteria were: (1) Adequate B-mode USG image data, (2) diagnosis of renal cortical necrosis by renal biopsy, and (3) availability of relevant clinical data. Exclusion criteria were: (1) substandard image quality, (2) lack of clinical data, (3) missing histopathological confirmation, and (4) history of CKD.
Data collection
Medical records and B-mode USG kidney images of all 23 patients with biopsy-proven postpartum RCN over the last 24 months were retrieved. Gray-scale B-mode kidney imaging was conducted using a 2-5 MHz convex array transducer by an experienced nephrologist before biopsy. USG kidney parameters assessed included kidney size, presence of hypoechoic rim, echogenicity, CMD, etc. Differentiation from the perinephric fluid collection: Perinephric fluid collections contrast with the hypoechoic cortical rim of RCN due to their extracapsular in location and lower echogenicity. All images were reviewed and confirmed by a radiologist at our hospital.
Statistical analysis
All analyses were performed using IBM Stata version 25 and Microsoft Excel 2011. Continuous variables were presented as means with standard deviations. Categorical variables were reported as numbers and percentages. Student’s test was used to assess significant differences in measurements. A probability of P < 0.05 was considered statistically significant.
Results
The mean age was 27.52 ± 4.62 years, ranging from 21 to 38 years. Seventeen patients were multiparous, and six were primiparous. Seven patients out of 23 experienced preterm delivery, while 12 underwent cesarean delivery. Twelve out of 23 patients had multiple risk factors. Seven had puerperal sepsis; six patients had atypical hemolytic uremic syndrome (aHUS); seven had placental abruption with intrauterine death (IUD); six had postpartum hemorrhage (PPH), nine had preeclampsia (seven complicated with placental abruption and two with PPH), one had hemolysis, elevated liver enzymes and low platelets (HELLP), two had lupus nephritis, one had thrombotic thrombocytopenic purpura (TTP), and one had eclampsia.
The B-mode USG-assessed parameters of 23 postpartum RCN patients are summarized in Table 1. Out of the 23 patients, hypoechoic rim was seen in 6 patients (26.1%) [Figures 1 and 2]. USG kidney timing ranged from 1.1 to 8 weeks. Kidney length varied from 9.0 to 10.8 cm. The mean postpartum USG kidney timing in patients with and without hypoechoic rim was 2.5 ± 0.77 and 4.81 ± 2.17 weeks, respectively. There was a significant difference in postpartum USG timing between patients with hypoechoic and without hypoechoic rim (P = 0.020). Mean kidney length on B-mode USG in patients with hypoechoic and without hypoechoic rim were 10.10 ± 0.52 cm and 9.576 ± 0.4024 cm, respectively. A significant difference in mean kidney length was noted between patients with and without a hypoechoic rim (P = 0.036) [Figure 3].
| B-mode USG parameters | Mean ± SD or % (n/23) |
|---|---|
| Postpartum USG kidney timing (wk) | 4.209 ± 2.16 |
| Length of kidney (cm) | 9.713 ± 0.48 |
| Loss of CMD | 65.2% (15/23) |
| Hypoechoic rim | 26.1% (6/23) |
| Physiological hydronephrosis | 73.9% (17/23) |
CMD: cortico-medullary differentiation. USG: ultrasonography.
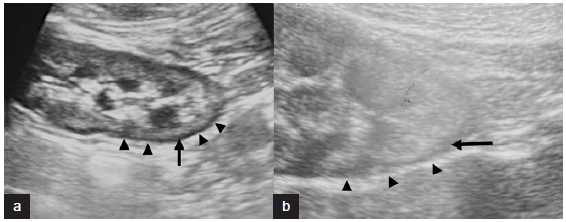
- B-mode ultrasonography (USG) images from two different patients (a) and (b) showing renal cortical rim sign. Black arrows show hypoechoic renal cortex which is seen in circumference of entire kidney in areas both closer and away from the ultrasound probe. Black arrowheads show echogenic layer outside the hypoechoic rim.
![Kidney biopsy findings. Complete cortical infarct with outline of glomerular vessels defined on (a) PAS and (b) Silver methenamine with large fibrin thrombi in glomerular lumen (green arrow in a). Afferent arteriole also shows fibrin thrombus (red arrow in a) [Periodic Acid Schiff (PAS) and silver methenamine 20x].](/content/170/2024/0/1/img/IJN_244_2024-g2.png)
- Kidney biopsy findings. Complete cortical infarct with outline of glomerular vessels defined on (a) PAS and (b) Silver methenamine with large fibrin thrombi in glomerular lumen (green arrow in a). Afferent arteriole also shows fibrin thrombus (red arrow in a) [Periodic Acid Schiff (PAS) and silver methenamine 20x].
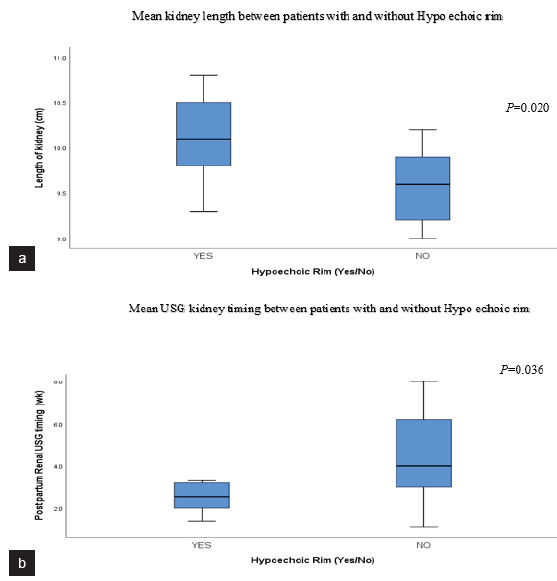
- B-mode US findings. (a) Box plot showing significant difference in mean kidney length between patients with and without hypoechoic rim. (b) Box plot showing significant difference in mean USG kidney timing in patients with and without hypoechoic rim. The mean USG kidney timing was significantly earlier in patients with sono-graphic hypoechoic rim sign (2.5 ± 0.77 wks. vs 4.81 ± 2.17 wks.). USG: ultrasonography.
Discussion
RCN is a rare, irreversible form of acute kidney injury (AKI). According to an old study from north India, the incidence of RCN was reported as 3.8%.4 However, a recent study indicated a progressive decline in RCN incidence.5 We have observed a significant number of cases in the past 2 years. This is due to the high footfall of obstetric AKI referrals to our hospital from the adjoining states. RCN is typically diagnosed using renal biopsy and through characteristic enhancement patterns on cross-sectional imaging such as CECT abdomen, MRI abdomen and CEUS. Biopsy remains the gold standard for diagnosing cortical necrosis; however, it is often avoided due to poor hemodynamic status and coagulopathy.5 Because acute cortical necrosis is uncommon, there is limited published information regarding its appearance on sonography. In our study, conventional B-mode USG showed hypoechoic cortex suggestive of RCN in around 26 % of biopsy-proven cases. Among 23 biopsy-proven RCN patients, hypoechoic rim suggestive of RCN was observed in 6 patients. The presence of a hypoechoic rim was significantly associated with the timing of USG after delivery. All the patients were dependent on dialysis at the time of undergoing USG kidney assessment.
RCN is usually bilateral due to underlying systemic etiologies. However, isolated unilateral cases have been reported.6 Isolated renal cortex necrosis is believed to result from intravascular thrombosis affecting interlobular and afferent arterioles. The arcuate arteries supply the cortico-medullary junction nephrons, while the sub-capsular nephrons receive vascular supply from a rich and extensive anastomotic extra renal network. These two regions of the kidney are usually spared from infarction in RCN.7 Causes of RCN can be broadly classified into obstetric (50-70%) or non-obstetric causes (20-30%). Obstetric complications include septic abortion, placental abruption, puerperal sepsis, PPH, and thrombotic microangiopathy (TMA) associated with pregnancy. Frimat et al. described the clinical course of 18 patients with RCN due to PPH.8
For the first time in literature, Sefczek et al. in 1984, described hypoechoic low-attenuation, circumferential band adjacent to the renal capsule on B-mode USG, corresponding to the histologic zone of cortical necrosis in RCN.9,10 The sonographic images obtained in our study also displayed this zone, appearing as a hypoechoic area adjacent to the capsule. Most renal diseases result in increased echogenicity of the cortex, with RCN reported to display a hypoechoic circumferential band on sonography. Due to lack of awareness of this sonographic finding and the rarity of these patients, we may have previously missed identifying the hypoechoic rim on USG kidney scans. However, with an increasing number of admissions for postpartum AKI, we have more accurately identified the hypoechoic cortical rim with assistance from radiologists. An important mimicker of sono-graphic hypoechoic rim was perinephric collection, which was differentiated by an anechoic rim surrounding the kidney and located extracapsularly11 and indirectly by real-time USG-guided renal biopsy of renal tissue retrieved from the hypoechoic region.
Spiesecke P et al.12 reported hypoechoic cortex using B-mode USG in around 33.33 % of RCN patients, compared to 26% reporting in our study. This variation in reporting of sonographic rim signs may be due to differences in USG timings after the onset of RCN. Pal et al. supported this observation, suggesting the hypoechoic rim appears early, prior to renal enlargement or cortical calcifications.13 In our study, the hypoechoic rim sign also correlated with USG timing and kidney length, suggesting the role of B-mode USG in early detection of the hypoechoic rim during disease progression.
CEUS assessment is a safe, easy, and efficacious investigative modality to identify patients with RCN from those with potentially reversible medical causes, but it is not widely available.14 While the sensitivity of sonographic hypoechoic rim is lower than that of contrast imaging finding, B-mode USG availability and lack of risk make it a valuable early feature of postpartum RCN. Our study’s strength is the sonographic hypoechoic rim reporting in a large case series of biopsy-proven RCN patients. Previous studies were either case reports or small case series.
The study’s shortcomings are the single-center retrospective study design with no control group and the patients’ non-availability of complete renal doppler records.
Conclusion
Despite lower sensitivity, the sonographic rim sign is an important diagnostic feature of acute renal cortical necrosis. It should be diligently sought early in the disease course, especially in cases of postpartum anuric AKI, using point-of-care ultrasonography.
Acknowledgment
The author sincerely thank Dr. D K Shukla, Ph.D., and Ex. ICMR for statistical analysis.
Conflicts of interest
There are no conflicts of interest.
References
- Acute kidney injury (AKI) In: Rodriguez A, Barraco R, Ivatury R, eds. Geriatric Trauma and Acute Care Surgery. Cham: Springer; 2018. p. :367-80.
- [Google Scholar]
- AJKD atlas of renal pathology: Cortical necrosis. Am J Kidney Dis Off J Natl Kidney Found. 2016;67:e27-8.
- [CrossRef] [Google Scholar]
- PoCUS in nephrology: A new tool to improve our diagnostic skills. Clin Kidney J. 2022;16:218-29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute renal cortical necrosis: A study of 113 patients. Ren Fail. 1994;16:37-47.
- [CrossRef] [PubMed] [Google Scholar]
- Changing picture of renal cortical necrosis in acute kidney injury in developing country. World J Nephrol. 2015;4:480-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Unilateral acute renal cortical necrosis (ACN) following skipping with a rope. Nephrol Dial Transplant. 2000;15:415-18.
- [CrossRef] [PubMed] [Google Scholar]
- Acute cortical necrosis: case report and review of the literature. Am J Med. 1974;56:110-8.
- [CrossRef] [PubMed] [Google Scholar]
- Renal cortical necrosis in postpartum hemorrhage: a case series. Am J Kidney Dis. 2016;68:50-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography of acute renal cortical necrosis. AJR Am J Roentgenol. 1984;142:553-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonography in acute kidney injury. POCUS J. 2022;7:35-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Perirenal fluid collection in renal parenchymal disease: The kidney sweat sign. AbdomRadiol (NY). 2017;42:1809-10.
- [CrossRef] [Google Scholar]
- Multiparametric ultrasound findings in acute kidney failure due to rare renal cortical necrosis. Sci Rep. 2021;11:2060.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sonographic rim in acute renal cortical necrosis. Int J Anat Radiol Surg. 2021;10:RC06-7.
- [CrossRef] [Google Scholar]
- Contrast enhanced ultrasound (CEUS) in the diagnosis of postpartum bilateral renal cortical necrosis: A case report and review of the literature. Abdom Imaging. 2014;39:550-3.
- [CrossRef] [PubMed] [Google Scholar]








